This is a preprint.
SIRPα controls CD47-dependent platelet clearance in mice and humans
- PMID: 38106070
- PMCID: PMC10723388
- DOI: 10.1101/2023.12.09.570874
SIRPα controls CD47-dependent platelet clearance in mice and humans
Abstract
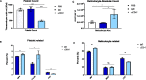
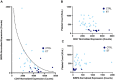
Over the last decade, more data has revealed that increased surface expression of the "don't eat me" CD47 protein on cancer cells plays a role in immune evasion and tumor progression, with CD47 blockade emerging as a new therapy in immuno-oncology. CD47 is critical in regulating cell homeostasis and clearance, as binding of CD47 to the inhibitory receptor SIRPα can prevent phagocytosis and macrophage-mediated cell clearance. The purpose of this study was to examine the role of the CD47-SIRPα signal in platelet homeostasis and clearance. Therapeutic reagents targeting the CD47-SIRPα axis are very promising for treatment of hematologic malignancies and solid tumors, but lead to transient anemia or thrombocytopenia in a subset of patients. We found that platelet homeostatic clearance is regulated through the CD47-SIRPα axis and that therapeutic blockade to disrupt this interaction in mice and in humans has a significant impact on platelet levels. Furthermore, we identified genetic variations at the SIRPA locus that impact platelet levels in humans such that higher SIRPA gene expression is associated with higher platelet levels. SIRPA expression at either end of the normal range may affect clinical outcomes of treatment with anti-CD47 therapy.
Conflict of interest statement
Conflict of interest declaration H.M.O., M.C.T., Y.Y.Y, and I.L.W. are co-inventors on pct/us2019/050650 which is related to this work. M.C.T., Y.Y.Y, and I.L.W. are co-inventors on PCT/US2020/015905 related to this work. M.C.T. and I.L.W. are co-inventors on a patent application (63/107,295) related to this work. M.C.T., M.S. and I.L.W. are co-inventors on a patent application (17/425,224) related to this work. I.L.W. is an inventor on U.S. patent 2019/0092873 A1 CD47, Targeted Therapies for the Treatment of Infectious Disease. I.L.W. is a cofounder, director, and stockholder in FortySeven Inc., a public company that was involved in CD47-based immunotherapy of cancer during this study but was acquired by Gilead. At the time of this submission, I.L.W. has no formal relationship with Gilead, and is engaged in co-founding a company dealing with atherosclerosis and CD47.
Figures




References
-
- Takimoto CH, Chao MP, Gibbs C, et al. The Macrophage ‘Do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol. 2019;30(3):486–489. - PubMed
-
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
