This is a preprint.
Brain-charting autism and attention deficit hyperactivity disorder reveals distinct and overlapping neurobiology
- PMID: 38106166
- PMCID: PMC10723556
- DOI: 10.1101/2023.12.06.23299587
Brain-charting autism and attention deficit hyperactivity disorder reveals distinct and overlapping neurobiology
Update in
-
Brain-Charting Autism and Attention-Deficit/Hyperactivity Disorder Reveals Distinct and Overlapping Neurobiology.Biol Psychiatry. 2025 Mar 1;97(5):517-530. doi: 10.1016/j.biopsych.2024.07.024. Epub 2024 Aug 14. Biol Psychiatry. 2025. PMID: 39128574
Abstract
Background: Autism and attention deficit hyperactivity disorder (ADHD) are heterogeneous neurodevelopmental conditions with complex underlying neurobiology. Despite overlapping presentation and sex-biased prevalence, autism and ADHD are rarely studied together, and sex differences are often overlooked. Normative modelling provides a unified framework for studying age-specific and sex-specific divergences in neurodivergent brain development.
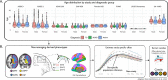
Methods: Here we use normative modelling and a large, multi-site neuroimaging dataset to characterise cortical anatomy associated with autism and ADHD, benchmarked against models of typical brain development based on a sample of over 75,000 individuals. We also examined sex and age differences, relationship with autistic traits, and explored the co-occurrence of autism and ADHD (autism+ADHD).
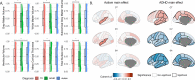
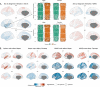
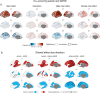
Results: We observed robust neuroanatomical signatures of both autism and ADHD. Overall, autistic individuals showed greater cortical thickness and volume localised to the superior temporal cortex, whereas individuals with ADHD showed more global effects of cortical thickness increases but lower cortical volume and surface area across much of the cortex. The autism+ADHD group displayed a unique pattern of widespread increases in cortical thickness, and certain decreases in surface area. We also found evidence that sex modulates the neuroanatomy of autism but not ADHD, and an age-by-diagnosis interaction for ADHD only.
Conclusions: These results indicate distinct cortical differences in autism and ADHD that are differentially impacted by age, sex, and potentially unique patterns related to their co-occurrence.
Conflict of interest statement
Disclosures EB reports consultancy work for Boehringer Ingelheim, Sosei Heptares, SR One, GlaxoSmithKline. EB, RAIB, JS, AFA-B are co-founders of Centile Bioscience. PAD receives research support from Biohaven Pharmaceuticals. M-C Lai has received editorial honorarium from SAGE Publications. RN reported receiving grants from Brain Canada, Hoffman La Roche, Otsuka Pharmaceuticals, and Maplight Therapeutics outside the submitted work. EA reported receiving grants from Roche and Anavex; receiving nonfinancial support from AMO Pharma and CRA-Simons Foundation; and receiving personal fees from Roche, Impel, Ono, and Quadrant outside the submitted work; in addition, EA had a patent for Anxiety Meter issued 14/755/084 (United States) and a patent for Anxiety Meter pending 2,895,954 (Canada) as well as receiving royalties from APPI and Springer. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures

References
-
- Lai M.-C., Lombardo M. V. & Baron-Cohen S. Autism. Lancet 383, 896–910 (2014). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
