This is a preprint.
Zika Virus NS1 Drives Tunneling Nanotube Formation for Mitochondrial Transfer, Enhanced Survival, Interferon Evasion, and Stealth Transmission in Trophoblasts
- PMID: 38106210
- PMCID: PMC10723532
- DOI: 10.21203/rs.3.rs-3674059/v1
Zika Virus NS1 Drives Tunneling Nanotube Formation for Mitochondrial Transfer, Enhanced Survival, Interferon Evasion, and Stealth Transmission in Trophoblasts
Update in
-
Zika virus NS1 drives tunneling nanotube formation for mitochondrial transfer and stealth transmission in trophoblasts.Nat Commun. 2025 Feb 20;16(1):1803. doi: 10.1038/s41467-025-56927-2. Nat Commun. 2025. PMID: 39979240 Free PMC article.
Abstract
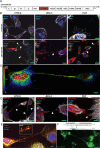
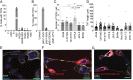
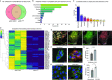
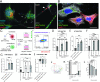
Zika virus (ZIKV) infection continues to pose a significant public health concern due to limited available preventive measures and treatments. ZIKV is unique among flaviviruses in its vertical transmission capacity (i.e., transmission from mother to fetus) yet the underlying mechanisms remain incompletely understood. Here, we show that both African and Asian lineages of ZIKV induce tunneling nanotubes (TNTs) in placental trophoblasts and multiple other mammalian cell types. Amongst investigated flaviviruses, only ZIKV strains trigger TNTs. We show that ZIKV-induced TNTs facilitate transfer of viral particles, proteins, and RNA to neighboring uninfected cells. ZIKV TNT formation is driven exclusively via its non-structural protein 1 (NS1); specifically, the N-terminal region (50 aa) of membrane-bound NS1 is necessary and sufficient for triggering TNT formation in host cells. Using affinity purification-mass spectrometry of cells infected with wild-type NS1 or non-TNT forming NS1 (pNS1ΔTNT) proteins, we found mitochondrial proteins are dominant NS1-interacting partners, consistent with the elevated mitochondrial mass we observed in infected trophoblasts. We demonstrate that mitochondria are siphoned via TNTs from healthy to ZIKV-infected cells, both homotypically and heterotypically, and inhibition of mitochondrial respiration reduced viral replication in trophoblast cells. Finally, ZIKV strains lacking TNT capabilities due to mutant NS1 elicited a robust antiviral IFN-λ 1/2/3 response, indicating ZIKV's TNT-mediated trafficking also allows ZIKV cell-cell transmission that is camouflaged from host defenses. Together, our findings identify a new stealth mechanism that ZIKV employs for intercellular spread among placental trophoblasts, evasion of antiviral interferon response, and the hijacking of mitochondria to augment its propagation and survival. Discerning the mechanisms of ZIKV intercellular strategies offers a basis for novel therapeutic developments targeting these interactions to limit its dissemination.
Conflict of interest statement
Competing interests IUM serves on the scientific advisory board of Luca Biologics.
Figures

References
-
- Duffy M. R. et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 360, 2536–2543 (2009). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

