Tensor radiomics: paradigm for systematic incorporation of multi-flavoured radiomics features
- PMID: 38106259
- PMCID: PMC10722050
- DOI: 10.21037/qims-23-163
Tensor radiomics: paradigm for systematic incorporation of multi-flavoured radiomics features
Abstract
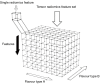
Background: Radiomics features hold significant value as quantitative imaging biomarkers for diagnosis, prognosis, and treatment response assessment. To generate radiomics features and ultimately develop signatures, various factors can be manipulated, including image discretization parameters (e.g., bin number or size), convolutional filters, segmentation perturbation, or multi-modality fusion levels. Typically, only one set of parameters is employed, resulting in a single value or "flavour" for each radiomics feature. In contrast, we propose "tensor radiomics" (TR) where tensors of features calculated using multiple parameter combinations (i.e., flavours) are utilized to optimize the creation of radiomics signatures.
Methods: We provide illustrative instances of TR implementation in positron emission tomography-computed tomography (PET-CT), magnetic resonance imaging (MRI), and CT by leveraging machine learning (ML) and deep learning (DL) methodologies, as well as reproducibility analyses: (I) to predict overall survival (OS) in lung cancer (CT) and head and neck cancer (PET-CT), TR was employed by varying bin sizes. This approach involved use of a hybrid deep neural network called 'TR-Net' and two ML-based techniques for combining different flavours. (II) TR was constructed by incorporating different segmentation perturbations and various bin sizes to classify the response of late-stage lung cancer to first-line immunotherapy using CT images. (III) In MRI of glioblastoma (GBM), TR was implemented to generate multi-flavour radiomics features, enabling enhanced analysis and interpretation. (IV) TR was employed via multiple PET-CT fusions in head and neck cancer. Flavours based on different fusions were created using Laplacian pyramids and wavelet transforms.
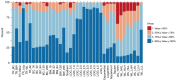
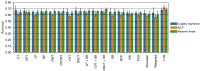
Results: Our findings demonstrated that TR outperformed conventional radiomics features in lung cancer CT and head and neck cancer PET-CT images, significantly enhancing OS prediction accuracy. TR also improved classification of lung cancer response to therapy and exhibited notable advantages in reproducibility compared to single-flavour features in MR imaging of GBM. Moreover, in head and neck cancer, TR through multiple PET-CT fusions exhibited improved performance in predicting OS.
Conclusions: We conclude that the proposed TR paradigm has significant potential to improve performance in different medical imaging tasks. By incorporating multiple flavours of radiomics features, TR overcomes limitations associated with individual features and shows promise in enhancing prognostic capabilities in clinical settings.
Keywords: Imaging biomarkers; image fusion; machine learning (ML); outcome/disease prediction; radiomics.
2023 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-163/coif). A.R. reports that this work was in part supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (No. RGPIN-2019-06467), the Canadian Institutes of Health Research (CIHR) Project Grants (Nos. PJT-162216 and PJT-173231), and the BC Cancer Foundation. H.Z. reports that this work was in part supported by the Swiss National Science Foundation Grant (No. SNRF 320030_176052). C.H. has received honoraria paid to self from Abbvie, Amgen, AstraZeneca, Bayer, BMS, Eisai, Jazz, Janssen, Merck, Novartis, Pfizer, Roche, Sanofi and research grants paid to the institution from AstraZeneca and Roche. A.R. and C.U. are cofounders of Ascinta technologies Inc. The other authors have no conflicts of interest to declare.
Figures






References
LinkOut - more resources
Full Text Sources
