Beyond Eosinophilic Esophagitis: Eosinophils in Gastrointestinal Disease-New Insights, "New" Diseases
- PMID: 38106480
- PMCID: PMC10723938
- DOI: 10.1093/jcag/gwad046
Beyond Eosinophilic Esophagitis: Eosinophils in Gastrointestinal Disease-New Insights, "New" Diseases
Abstract
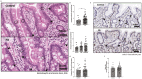
Functional dyspepsia (FD) is a highly prevalent disorder. Upper endoscopy is normal, and according to the Rome IV criteria, there is no established pathology. Data accumulated over the last 15 years has challenged the notion FD is free of relevant pathology, and in particular, increased duodenal eosinophils have been observed. Intestinal eosinophils play important roles in microbial defence, immune regulation, tissue regeneration and remodelling, and maintaining tissue homeostasis and metabolism; degranulation of eosinophils releases toxic granule products (e.g., major basic protein, eosinophil-derived neurotoxin) which can damage nerves. Normal cut-offs for eosinophil infiltration into the duodenum histologically are less than five eosinophils per high power field (<25 per five high power fields). In clinical practice there is evidence that pathologically increased intestinal eosinophils may often be overlooked. In a meta-analysis duodenal eosinophils were significantly increased in FD although there was substantial heterogeneity; degranulation of duodenal eosinophils was also significantly higher in FD without significant heterogeneity. In addition, increased duodenal permeability, systemic immune activation, and an altered mucosa-associated duodenal microbiome have been identified that may help explain why symptoms arise, often occur after food with exposure to food antigens, and typically fluctuate. Several potentially reversible risk factors for FD have now been identified. We evaluate the current evidence linking duodenal microinflammation and immune activation with FD and disorders of gut-brain interactions that overlap with FD. We propose a two-hit disease model for eosinophilic functional dyspepsia (EoFD) with management implications.
Keywords: Eosinophils; food antigens; functional dyspepsia; mast cells; microbiome.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Canadian Association of Gastroenterology.
Conflict of interest statement
Nicholas J. Talley delivered the RD McKenna Memorial Lecture in 2023. He reports non-financial support from: Norgine (2021) (IBS interest group), personal fees from Allakos (gastroduodenal eosinophilic disease) (2021), Bayer (IBS) (2020), Planet Innovation (Gas capsule IBS) (2020), twoXAR Viscera Labs, (USA 2021) (IBS-diarrhoea), Dr Falk Pharma (2020) (EoE), Sanofi-aventis, Glutagen (2020) (celiac disease), IsoThrive (2021) (esophageal microbiome), BluMaiden (microbiome advisory board) (2021), Rose Pharma (IBS) (2021), Intrinsic Medicine (2022) (human milk oligosaccharide), Comvita Mānuka Honey (2021) (digestive health), Astra Zeneca (2022), outside the submitted work. In addition, Dr. Talley has a patent Nepean Dyspepsia Index (NDI) 1998, Biomarkers of IBS licensed, a patent Licensing Questionnaires Talley Bowel Disease Questionnaire licensed to Mayo/Talley, a patent Nestec European Patent licensed, and a patent Singapore Provisional Patent “Microbiota Modulation of BDNF Tissue Repair Pathway” issued, “Diagnostic marker for functional gastrointestinal disorders” Australian Provisional Patent Application 2021901692. Committees: OzSage; NHMRC Principal Committee (Research Committee) Asia Pacific Association of Medical Journal Editors, Rome V Working Team Member (Gastroduodenal Committee), International Plausibility Project Co-Chair (Rome Foundation funded), COVID-19 vaccine forum member (by invitation only). Community group: Advisory Board, IFFGD (International Foundation for Functional GI Disorders), AusEE. Editorial: Medical Journal of Australia (Emeritus Editor in Chief), Mayo Clinic Proceedings (Assoc Ed), Up to Date (Section Editor), Precision and Future Medicine, Sungkyunkwan University School of Medicine, South Korea, Med (Journal of Cell Press), Gastroenterology (Advisory Board). Simon Keely reports personal fees from Gossamer Bio, Anatara Lifescience, Immuron Ltd, Microba Life Science. He has received research support funding from Viscera Labs, Microba Life Science, Anatara Lifesciences and Gossamer Bio. He is currently funded by National Health and Medical Research Council (NHMRC) and Australian Department of Defence grants. He is listed as inventor on Diagnostic marker for functional gastrointestinal disorders” Australian Provisional Patent Application 2021901692.
Figures


References
-
- Aziz, Imran, Palsson Olafur S., Törnblom Hans, Sperber Ami D., Whitehead William E., and Simrén Magnus.. “Epidemiology, Clinical Characteristics, and Associations for SYMPTOM-BASED ROME IV Functional Dyspepsia in Adults in the USA, Canada, and the UK: A Cross-Sectional Population-Based Study.” Lancet Gastroenterol Hepatol 3, no. 4 (2018): 252–62. 10.1016/S2468-1253(18)30003-7 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
