Development of neonatal-specific sequences for portable ultralow field magnetic resonance brain imaging: a prospective, single-centre, cohort study
- PMID: 38106560
- PMCID: PMC10725077
- DOI: 10.1016/j.eclinm.2023.102253
Development of neonatal-specific sequences for portable ultralow field magnetic resonance brain imaging: a prospective, single-centre, cohort study
Abstract
Background: Magnetic Resonance (MR) imaging is key for investigation of suspected newborn brain abnormalities. Access is limited in low-resource settings and challenging in infants needing intensive care. Portable ultralow field (ULF) MRI is showing promise in bedside adult brain imaging. Use in infants and children has been limited as brain-tissue composition differences necessitate sequence modification. The aim of this study was to develop neonatal-specific ULF structural sequences and test these across a range of gestational maturities and pathologies to inform future validation studies.
Methods: Prospective cohort study within a UK neonatal specialist referral centre. Infants undergoing 3T MRI were recruited for paired ULF (64mT) portable MRI by convenience sampling from the neonatal unit and post-natal ward. Key inclusion criteria: 1) Infants with risk or suspicion of brain abnormality, or 2) preterm and term infants without suspicion of major genetic, chromosomal or neurological abnormality. Exclusions: presence of contra-indication for MR scanning. ULF sequence parameters were optimised for neonatal brain-tissues by iterative and explorative design. Neuroanatomic and pathologic features were compared by unblinded review, informing optimisation of subsequent sequence generations in a step-wise manner. Main outcome: visual identification of healthy and abnormal brain tissues/structures. ULF MR spectroscopy, diffusion, susceptibility weighted imaging, arteriography, and venography require pre-clinical technical development and have not been tested.
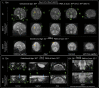
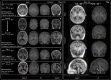
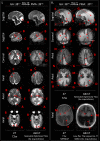
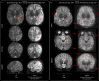
Findings: Between September 23, 2021 and October 25, 2022, 102 paired scans were acquired in 87 infants; 1.17 paired scans per infant. Median age 9 days, median postmenstrual age 40+2 weeks (range: 31+3-53+4). Infants had a range of intensive care requirements. No adverse events observed. Optimised ULF sequences can visualise key neuroanatomy and brain abnormalities. In finalised neonatal sequences: T2w imaging distinguished grey and white matter (7/7 infants), ventricles (7/7), pituitary tissue (5/7), corpus callosum (7/7) and optic nerves (7/7). Signal congruence was seen within the posterior limb of the internal capsule in 10/11 infants on finalised T1w scans. In addition, brain abnormalities visualised on ULF optimised sequences have similar MR signal patterns to 3T imaging, including injury secondary to infarction (6/6 infants on T2w scans), hypoxia-ischaemia (abnormal signal in basal ganglia, thalami and white matter 2/2 infants on T2w scans, cortical highlighting 1/1 infant on T1w scan), and congenital malformations: polymicrogyria 3/3, absent corpus callosum 2/2, and vermian hypoplasia 3/3 infants on T2w scans. Sequences are susceptible to motion corruption, noise, and ULF artefact. Non-identified pathologies were small or subtle.
Interpretation: On unblinded review, optimised portable MR can provide sufficient contrast, signal, and resolution for neuroanatomical identification and detection of a range of clinically important abnormalities. Blinded validation studies are now warranted.
Funding: The Bill and Melinda Gates Foundation, the MRC, the Wellcome/EPSRC Centre for Medical Engineering, the MRC Centre for Neurodevelopmental Disorders, and the National Institute for Health Research (NIHR) Biomedical Research Centres based at Guy's and St Thomas' and South London & Maudsley NHS Foundation Trusts and King's College London.
Keywords: Intensive care; Low field; Magnetic resonance imaging; Neonatal; Portable.
© 2023 The Author(s).
Conflict of interest statement
PC is supported by the Medical Research Council Centre for Neurodevelopmental Disorders [MR/N026063/1] to undertake this work and received an educational stipend from ISMRM to attend the international ISMRM conference and present this work. FP was employed by the Guy′s & St. Thomas′ NHS Foundation Trust & King’s College London as a senior MR physicist at study outset, during experimental design and initial participant recruitment, he is now a senior clinical scientist employed by Hyperfine Inc., since May 2022–drawing a salary, shares and stock options. RT is an MR sequence developer employed by Hyperfine Inc., and is a holder of shares and stock option of Hyperfine Inc. JOM has institutional funding from the Bill & Melinda Gates Foundation Consortium grant to support research work by his group in neurodevelopment, including data from conventional 3T MRI and Hyperfine scanners–this grant is focused on image analysis and is not commercially sponsored. SW has received funding from the Bill & Melinda Gates Foundation for attendance and travel to sites for training and knowledge exchange during the development and delivery of this project. TA is supported by the UK MRC for a Translational support fellowship [MR/V036874/1] for personal salary, and funding for 3T MRI scans, the MRC Centre for Neurodevelopmental Disorders, King’s College London [MR/N026063/1] for administrative support, funding, and a Clinician Scientist Fellowship [MR/P008712/1]—for personal salary and funding for 3T MRI scans. TA is supported by the EPSRC UK Network grant, co-investigator [EP/W035154/1]. DE and JVH: The Hyperfine machine (Swoop® MR System) was provided by the Bill and Melinda Gates Foundation as part of the Unity Consortium.
Figures

References
-
- Schreglmann M., Ground A., Vollmer B., Johnson M.J. Systematic review: long-term cognitive and behavioural outcomes of neonatal hypoxic–ischaemic encephalopathy in children without cerebral palsy. Acta Paediatr. 2020;109:20–30. - PubMed
-
- Tonks J., Cloke G., Lee-Kelland R., et al. Attention and visuo-spatial function in children without cerebral palsy who were cooled for neonatal encephalopathy: a case-control study. Brain Inj. 2019;33:894–898. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

