Efficacy and Safety of Iptacopan in Patients With C3 Glomerulopathy
- PMID: 38106570
- PMCID: PMC10719607
- DOI: 10.1016/j.ekir.2023.09.017
Efficacy and Safety of Iptacopan in Patients With C3 Glomerulopathy
Abstract
Introduction: Complement 3 glomerulopathy (C3G) is a rare inflammatory kidney disease mediated by dysregulation of the alternative complement pathway. No targeted therapy exists for this aggressive glomerulonephritis. Efficacy, safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) (measured by complement biomarkers) of iptacopan were assessed in patients with C3G.
Methods: In this phase 2, multicenter, open-label, single-arm, nonrandomized study, adults with biopsy-proven, native kidney C3G (native cohort) and kidney transplant recipients with C3G recurrence (recurrent kidney transplant [KT] cohort) received iptacopan twice daily (bid) for 84 days (days 1-21: 10-100 mg; days 22-84: 200 mg). The primary end point was the urine protein-to-creatinine ratio (UPCR; native cohort) and the change in the C3 deposit score of kidney biopsy (recurrent KT cohort). The complement pathway measures included Wieslab assay, soluble C5b9, and serum C3 levels.
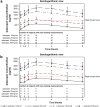
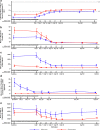
Results: A total of 27 patients (16 native cohort and 11 recurrent KT cohort) were enrolled and all completed the study. In the native cohort, UPCR levels decreased by 45% from baseline to week 12 (P = 0.0003). In the recurrent KT cohort, the median C3 deposit score decreased by 2.50 (scale: 0-12) on day 84 versus baseline (P = 0.03). Serum C3 levels were normalized in most patients; complement hyperactivity observed pretreatment was reduced. Severe adverse events (AEs) included post-biopsy hematuria and hyperkalemia. No deaths occurred during the study.
Conclusion: Iptacopan resulted in statistically significant and clinically important reductions in UPCR and normalization of serum C3 levels in the native cohort and reduced C3 deposit scores in the recurrent KT cohort with favorable safety and tolerability. (ClinicalTrials.gov identifier: NCT03832114).
Keywords: complement 3 glomerulopathy; inflammatory kidney disease; iptacopan; kidney transplant; urine protein-to-creatinine ratio.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

