Chondroprotective effects of Protaetia brevitarsis seulensis larvae as an edible insect on osteoarthritis in mice
- PMID: 38107146
- PMCID: PMC10724628
- DOI: 10.1002/fsn3.3706
Chondroprotective effects of Protaetia brevitarsis seulensis larvae as an edible insect on osteoarthritis in mice
Abstract
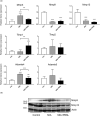
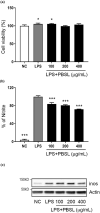
Osteoarthritis (OA) is a common chronic joint inflammatory disease characterized by progressive destruction of the articular cartilage, bone remodeling, and excessive chronic pain. Most therapeutic approaches do not rescue the progression of OA effectively or provide relief of symptoms. Protaetia brevitarsis seulensis larva (PBSL), which is attracting attention, is an edible insect with very high nutritional value and herbal medicine for the treatment of blood stasis, hepatic disease, and various inflammatory diseases. However, the effect of PBSL on OA has not yet been investigated. This study aimed to demonstrate the effects of PBSL water extract on the progression of OA using monosodium iodoacetate (MIA)-induced mice and SW1353 chondrocytes or murine macrophages. We injected MIA into the intraarticular area of mice following pretreatment with either saline or PBSL (200 mg/kg) for 2 weeks, and then locomotor activity, microcomputed tomography and histopathological analysis, quantitative reverse transcriptase-polymerase chain reaction analysis, and western blot analysis were performed. To determine the molecular effects of PBSL, we used interleukin-1β (IL-1β)-induced SW1353 chondrosarcoma or lipopolysaccharide (LPS)-stimulated macrophages. Pretreatment with PBSL diminished the symptoms of OA. Physical activity, articular cartilage damage, and the generation of microfractures were rescued by pretreatment with PBSL in the mouse model. Pretreatment with PBSL suppressed the progress of OA through the regulation of articular cartilage degradation genes and inflammation in both in vivo and in vitro models. Our results demonstrated that PBSL has value as edible insect that can be used in the development of functional foods for OA.
Keywords: Protaetia brevitarsis seulensis larva; chondroprotective effects; edible insect; osteoarthritis.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Alsalem, M. , Haddad, M. , Altarifi, A. , Aldossary, S. A. , Kalbouneh, H. , Abojaradeh, A. M. , & El‐Salem, K. (2020). Impairment in locomotor activity as an objective measure of pain and analgesia in a rat model of osteoarthritis. Experimental and Therapeutic Medicine, 20(6), 165. 10.3892/etm.2020.9294 - DOI - PMC - PubMed
-
- Cicko, S. , Grimm, M. , Ayata, K. , Beckert, J. , Meyer, A. , Hossfeld, M. , Zissel, G. , Idzko, M. , & Müller, T. (2015). Uridine supplementation exerts anti‐inflammatory and anti‐fibrotic effects in an animal model of pulmonary fibrosis. Respiratory Research, 16(1), 105. 10.1186/s12931-015-0264-9 - DOI - PMC - PubMed
-
- Cooper, C. , Chapurlat, R. , Al‐Daghri, N. , Herrero‐Beaumont, G. , Bruyère, O. , Rannou, F. , Roth, R. , Uebelhart, D. , & Reginster, J. Y. (2019). Safety of oral non‐selective non‐steroidal anti‐inflammatory drugs in osteoarthritis: What does the literature say? Drugs & Aging, 36(Suppl 1), 15–24. 10.1007/s40266-019-00660-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

