Enabling synthesis in fragment-based drug discovery (FBDD): microscale high-throughput optimisation of the medicinal chemist's toolbox reactions
- PMID: 38107176
- PMCID: PMC10718589
- DOI: 10.1039/d3md00495c
Enabling synthesis in fragment-based drug discovery (FBDD): microscale high-throughput optimisation of the medicinal chemist's toolbox reactions
Abstract
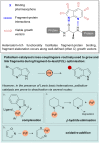
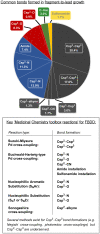
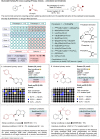
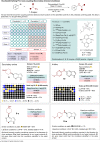
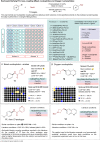
Miniaturised high-throughput experimentation (HTE) is widely employed in industrial and academic laboratories for rapid reaction optimisation using material-limited, multifactorial reaction condition screening. In fragment-based drug discovery (FBDD), common toolbox reactions such as the Suzuki-Miyaura and Buchwald-Hartwig cross couplings can be hampered by the fragment's intrinsic heteroatom-rich pharmacophore which is required for ligand-protein binding. At Astex, we are using microscale HTE to speed up reaction optimisation and prevent target down-prioritisation. By identifying catalyst/base/solvent combinations which tolerate unprotected heteroatoms we can rapidly optimise key cross-couplings and expedite route design by avoiding superfluous protecting group manipulations. However, HTE requires extensive upfront training, and this modern automated synthesis technique largely differs to the way organic chemists are traditionally trained. To make HTE accessible to all our synthetic chemists we have developed a semi-automated workflow enabled by pre-made 96-well screening kits, rapid analytical methods and in-house software development, which is empowering chemists at Astex to run HTE screens independently with minimal training.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
C. T., D. B., G. C., B. D. C., C. G.-J., R. J. H., C. N. J., S. W., and R. G. are all employees of Astex Pharmaceuticals. Y. O. is an employee of Otsuka Pharmaceutical Co., Ltd. and was on secondment to Astex Pharmaceuticals when this work was carried out.
Figures




References
-
- Murray C. W. Newell D. R. Angibaud P. MedChemComm. 2019;10:1509–1511. doi: 10.1039/C9MD90044F. - DOI
-
- Canon J. Rex K. Saiki A. Y. Mohr C. Cooke K. Bagal D. Gaida K. Holt T. Knutson C. G. Koppada N. Lanman B. A. Werner J. Rapaport A. S. San Miguel T. Ortiz R. Osgood T. Sun J.-R. Zhu X. McCarter J. D. Volak L. P. Houk B. E. Fakih M. G. O'Neil B. H. Price T. J. Falchook G. S. Desai J. Kuo J. Govindan R. Hong D. S. Ouyang W. Henary H. Arvedson T. Cee V. J. Lipford J. R. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources

