Rationale and design of the Children's Oncology Group study AAML1831 integrated cardiac substudies in pediatric acute myeloid leukemia therapy
- PMID: 38107263
- PMCID: PMC10722184
- DOI: 10.3389/fcvm.2023.1286241
Rationale and design of the Children's Oncology Group study AAML1831 integrated cardiac substudies in pediatric acute myeloid leukemia therapy
Abstract
Background: Pediatric acute myeloid leukemia (AML) therapy is associated with substantial short- and long-term treatment-related cardiotoxicity mainly due to high-dose anthracycline exposure. Early left ventricular systolic dysfunction (LVSD) compromises anthracycline delivery and is associated with inferior event-free and overall survival in de novo pediatric AML. Thus, effective cardioprotective strategies and cardiotoxicity risk predictors are critical to optimize cancer therapy delivery and enable early interventions to prevent progressive LVSD. While dexrazoxane-based cardioprotection reduces short-term cardiotoxicity without compromising cancer survival, liposomal anthracycline formulations have the potential to mitigate cardiotoxicity while improving antitumor efficacy. This overview summarizes the rationale and methodology of cardiac substudies within AAML1831, a randomized Children's Oncology Group Phase 3 study of CPX-351, a liposomal formulation of daunorubicin and cytarabine, in comparison with standard daunorubicin/cytarabine with dexrazoxane in the treatment of de novo pediatric AML.
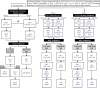
Methods/design: Children (age <22 years) with newly diagnosed AML were enrolled and randomized to CPX-351-containing induction 1 and 2 (Arm A) or standard daunorubicin and dexrazoxane-containing induction (Arm B). Embedded cardiac correlative studies aim to compare the efficacy of this liposomal anthracycline formulation to dexrazoxane for primary prevention of cardiotoxicity by detailed core lab analysis of standardized echocardiograms and serial cardiac biomarkers throughout AML therapy and in follow-up. In addition, AAML1831 will assess the ability of early changes in sensitive echo indices (e.g., global longitudinal strain) and cardiac biomarkers (e.g., troponin and natriuretic peptides) to predict subsequent LVSD. Finally, AAML1831 establishes expert consensus-based strategies in cardiac monitoring and anthracycline dose modification to balance the potentially competing priorities of cardiotoxicity reduction with optimal leukemia therapy.
Discussion: This study will inform diagnostic, prognostic, preventative, and treatment strategies regarding cardiotoxicity during pediatric AML therapy. Together, these measures have the potential to improve leukemia-free and overall survival and long-term cardiovascular health in children with AML. Clinical trial registration: https://clinicaltrials.gov/, identifier NCT04293562.
Keywords: CPX-351; cardiac biomarkers; cardiotoxicity; dexrazoxane; liposomal anthracycline; pediatric acute myeloid leukemia (AML); risk prediction.
© 2023 Leger, Robison, Narayan, Smith, Tsega, Chung, Daniels, Chen, Englefield, Demissei, Lefebvre, Morrow, Dizon, Gerbing, Pabari, Getz, Aplenc, Pollard, Chow, Tang, Border, Sachdeva, Alonzo, Kolb, Cooper and Ky.
Conflict of interest statement
KL, Jazz Pharmaceuticals (consulting, advisory board participation); KL and EC, Abbott Diagnostics (research funding). The reviewer YR declared a shared committee COG with the authors to the handling editor. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Getz KD, Sung L, Alonzo TA, Leger KJ, Gerbing RB, Pollard JA, et al. Effect of dexrazoxane on left ventricular systolic function and treatment outcomes in patients with acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. (2020) 38(21):2398–406. 10.1200/JCO.19.02856 - DOI - PMC - PubMed
-
- Getz KD, Sung L, Ky B, Gerbing RB, Leger KJ, Leahy AB, et al. Occurrence of treatment-related cardiotoxicity and its impact on outcomes among children treated in the AAML0531 clinical trial: a report from the Children’s Oncology Group. J Clin Oncol. (2019) 37(1):12–21. 10.1200/JCO.18.00313 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

