Navigating the Expansive Landscapes of Soft Materials: A User Guide for High-Throughput Workflows
- PMID: 38107416
- PMCID: PMC10722570
- DOI: 10.1021/acspolymersau.3c00025
Navigating the Expansive Landscapes of Soft Materials: A User Guide for High-Throughput Workflows
Abstract
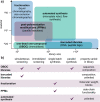
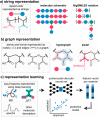
Synthetic polymers are highly customizable with tailored structures and functionality, yet this versatility generates challenges in the design of advanced materials due to the size and complexity of the design space. Thus, exploration and optimization of polymer properties using combinatorial libraries has become increasingly common, which requires careful selection of synthetic strategies, characterization techniques, and rapid processing workflows to obtain fundamental principles from these large data sets. Herein, we provide guidelines for strategic design of macromolecule libraries and workflows to efficiently navigate these high-dimensional design spaces. We describe synthetic methods for multiple library sizes and structures as well as characterization methods to rapidly generate data sets, including tools that can be adapted from biological workflows. We further highlight relevant insights from statistics and machine learning to aid in data featurization, representation, and analysis. This Perspective acts as a "user guide" for researchers interested in leveraging high-throughput screening toward the design of multifunctional polymers and predictive modeling of structure-property relationships in soft materials.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
Publication types
LinkOut - more resources
Full Text Sources
