Minimally Invasive Murine Laryngoscopy for Close-Up Imaging of Laryngeal Motion during Breathing and Swallowing
- PMID: 38108389
- PMCID: PMC11101017
- DOI: 10.3791/66089
Minimally Invasive Murine Laryngoscopy for Close-Up Imaging of Laryngeal Motion during Breathing and Swallowing
Abstract
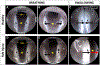
The larynx is an essential organ in mammals with three primary functions - breathing, swallowing, and vocalizing. A wide range of disorders are known to impair laryngeal function, which results in difficulty breathing (dyspnea), swallowing impairment (dysphagia), and/or voice impairment (dysphonia). Dysphagia, in particular, can lead to aspiration pneumonia and associated morbidity, recurrent hospitalization, and early mortality. Despite these serious consequences, existing treatments for laryngeal dysfunction are largely aimed at surgical and behavioral interventions that unfortunately do not typically restore normal laryngeal function, thus highlighting the urgent need for innovative solutions. To bridge this gap, we have been developing an experimental endoscopic approach to investigate laryngeal dysfunction in murine (i.e., mouse and rat) models. However, endoscopy in rodents is quite challenging due to their small size relative to current endoscope technology, anatomical differences in the upper airway, and the necessity for anesthesia to optimally access the larynx. Here, we describe a novel transoral laryngoscopy approach that permits close-up, unobstructed video imaging of laryngeal motion in mice and rats. Critical steps in the protocol include precise anesthesia management (to prevent overdosing that abolishes swallowing and/or risks respiratory distress-related mortality) and micromanipulator control of the endoscope (for stable video recording of laryngeal motion by a single researcher for subsequent quantification). Importantly, the protocol can be performed over time in the same animals to study the impact of various pathological conditions specifically on laryngeal function. A novel advantage of this protocol is the ability to visualize airway protection during swallowing, which is not possible in humans due to epiglottic inversion over the laryngeal inlet that obstructs the glottis from view. Rodents therefore provide a unique opportunity to specifically investigate the mechanisms of normal versus pathological laryngeal airway protection for the ultimate purpose of discovering treatments to effectively restore normal laryngeal function.
Conflict of interest statement
DISCLOSURES:
The authors have no conflicts of interest to declare.
Figures







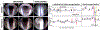

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
