Modeling Cataract Surgery in Mice
- PMID: 38108456
- PMCID: PMC10981495
- DOI: 10.3791/66050
Modeling Cataract Surgery in Mice
Abstract
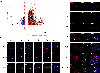
Cataract surgery (CS) is an effective treatment for cataracts, a major cause of visual disability worldwide. However, CS leads to ocular inflammation, and in the long term, it can result in posterior capsular opacification (PCO) and/or lens dislocation driven by the post-surgical overgrowth of lens epithelial cells (LECs) and their conversion to myofibroblasts and/or aberrant fiber cells. However, the molecular mechanisms by which CS results in inflammation and PCO are still obscure because most in vitro models do not recapitulate the wound healing response of LECs seen in vivo, while traditional animal models of cataract surgery, such as rabbits, do not allow the genetic manipulation of gene expression to test mechanisms. Recently, our laboratory and others have successfully used genetically modified mice to study the molecular mechanisms that drive the induction of proinflammatory signaling and LEC epithelial to mesenchymal transition, leading to new insight into PCO pathogenesis. Here, we report the established protocol for modeling cataract surgery in mice, which allows for robust transcriptional profiling of the response of LECs to lens fiber cell removal via RNAseq, the evaluation of protein expression by semi-quantitative immunofluorescence, and the use of modern mouse genetics tools to test the function of genes that are hypothesized to participate in the pathogenesis of acute sequelae like inflammation as well as the later conversion of LECs to myofibroblasts and/or aberrant lens fiber cells.
Figures

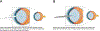


References
-
- Wormstone IM, Wormstone YM, Smith AJO, Eldred JA Posterior capsule opacification: What’s in the bag? Prog Retin Eye Res. 82, 100905 (2021). - PubMed
-
- Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS Cataracts. Lancet. 390 (10094), 600–612 (2017). - PubMed
-
- Khairallah M et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 56 (11), 6762–6769 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
