De novo design of high-affinity binders of bioactive helical peptides
- PMID: 38109936
- PMCID: PMC10849960
- DOI: 10.1038/s41586-023-06953-1
De novo design of high-affinity binders of bioactive helical peptides
Abstract
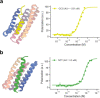
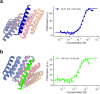
Many peptide hormones form an α-helix on binding their receptors1-4, and sensitive methods for their detection could contribute to better clinical management of disease5. De novo protein design can now generate binders with high affinity and specificity to structured proteins6,7. However, the design of interactions between proteins and short peptides with helical propensity is an unmet challenge. Here we describe parametric generation and deep learning-based methods for designing proteins to address this challenge. We show that by extending RFdiffusion8 to enable binder design to flexible targets, and to refining input structure models by successive noising and denoising (partial diffusion), picomolar-affinity binders can be generated to helical peptide targets by either refining designs generated with other methods, or completely de novo starting from random noise distributions without any subsequent experimental optimization. The RFdiffusion designs enable the enrichment and subsequent detection of parathyroid hormone and glucagon by mass spectrometry, and the construction of bioluminescence-based protein biosensors. The ability to design binders to conformationally variable targets, and to optimize by partial diffusion both natural and designed proteins, should be broadly useful.
© 2023. The Author(s).
Conflict of interest statement
D.B., S.V.T., P.J.Y.L., P.V., I.D.L., A.N.H., D.J., E.H., A.H.-W.Y., H.-H.H., J.L.W., M.J.M., N.R.B. and G.R.L. are inventors on a provisional patent application submitted by the University of Washington for the design and composition of the proteins created in this study.
Figures