Melatonin supplementation does not alter vascular function or oxidative stress in healthy normotensive adults on a high sodium diet
- PMID: 38110301
- PMCID: PMC10727961
- DOI: 10.14814/phy2.15896
Melatonin supplementation does not alter vascular function or oxidative stress in healthy normotensive adults on a high sodium diet
Abstract
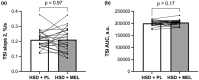
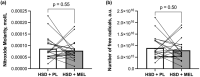
High sodium diets (HSD) can cause vascular dysfunction, in part due to increases in reactive oxygen species (ROS). Melatonin reduces ROS in healthy and clinical populations and may improve vascular function. The purpose was to determine the effect of melatonin supplementation on vascular function and ROS during 10 days of a HSD. We hypothesized that melatonin supplementation during a HSD would improve vascular function and decrease ROS levels compared to HSD alone. Twenty-seven participants (13 M/14 W, 26.7 ± 2.9 years, BMI: 23.6 ± 2.0 kg/m2 , BP: 110 ± 9/67 ± 7 mmHg) were randomized to a 10-day HSD (6900 mg sodium/d) supplemented with either 10 mg of melatonin (HSD + MEL) or a placebo (HSD + PL) daily. Brachial artery flow-mediated dilation, a measure of macrovascular function, (HSD + PL: 7.1 ± 3.8%; HSD + MEL: 6.7 ± 3.4%; p = 0.59) and tissue oxygenation index (TSI) reperfusion rate, a measure of microvascular reactivity, (HSD + PL: 0.21 ± 0.06%/s; HSD + MEL: 0.21 ± 0.08%/s; p = 0.97) and TSI area under the curve (HSD + PL: 199899 ± 10,863 a.u.; HSD + MEL: 20315 ± 11,348 a.u.; p = 0.17) were similar at the end of each condition. Neither nitroxide molarity (HSD + PL: 7.8 × 10-5 ± 4.1 × 10-5 mol/L; HSD + MEL: 8.7 × 10-5 ± 5.1 × 10-5 mol/L; p = 0.55) nor free radical number (HSD + PL: 8.0 × 1015 ± 4.4 × 1015 ; HSD + MEL: 9.0 × 1015 ± 4.9 × 1015 ; p = 0.51) were different between conditions. Melatonin supplementation did not alter vascular function or ROS levels while on a HSD in this sample of young healthy normotensive adults.
Keywords: NIRS; brachial artery FMD; melatonin; oxidative stress; sodium.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Figures

References
-
- Acuna‐Castroviejo, D. , Escames, G. , Venegas, C. , Diaz‐Casado, M. E. , Lima‐Cabello, E. , Lopez, L. C. , Rosales‐Corral, S. , Tan, D. X. , & Reiter, R. J. (2014). Extrapineal melatonin: Sources, regulation, and potential functions. Cellular and Molecular Life Sciences, 71(16), 2997–3025. - PMC - PubMed
-
- Altun, A. , Yaprak, M. , Aktoz, M. , Vardar, A. , Betul, U. A. , & Ozbay, G. (2002). Impaired nocturnal synthesis of melatonin in patients with cardiac syndrome X. Neuroscience Letters, 327(2), 143–145. - PubMed
-
- Ancoli‐Israel, S. , Martin, J. L. , Blackwell, T. , Buenaver, L. , Liu, L. , Meltzer, L. J. , Sadeh, A. , Spira, A. P. , & Taylor, D. J. (2015). The SBSM guide to actigraphy monitoring: Clinical and research applications. Behavioral Sleep Medicine, 13(Suppl 1), S4–S38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

