Targeting S100A9 protein affects mTOR-ER stress signaling and increases venetoclax sensitivity in Acute Myeloid Leukemia
- PMID: 38110349
- PMCID: PMC10728073
- DOI: 10.1038/s41408-023-00962-z
Targeting S100A9 protein affects mTOR-ER stress signaling and increases venetoclax sensitivity in Acute Myeloid Leukemia
Abstract
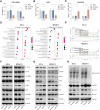
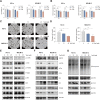
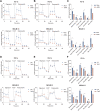
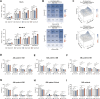
Acute Myeloid Leukemia (AML) is a heterogeneous disease with limited treatment options and a high demand for novel targeted therapies. Since myeloid-related protein S100A9 is abundantly expressed in AML, we aimed to unravel the therapeutic impact and underlying mechanisms of targeting both intracellular and extracellular S100A9 protein in AML cell lines and primary patient samples. S100A9 silencing in AML cell lines resulted in increased apoptosis and reduced AML cell viability and proliferation. These therapeutic effects were associated with a decrease in mTOR and endoplasmic reticulum stress signaling. Comparable results on AML cell proliferation and mTOR signaling could be observed using the clinically available S100A9 inhibitor tasquinimod. Interestingly, while siRNA-mediated targeting of S100A9 affected both extracellular acidification and mitochondrial metabolism, tasquinimod only affected the mitochondrial function of AML cells. Finally, we found that S100A9-targeting approaches could significantly increase venetoclax sensitivity in AML cells, which was associated with a downregulation of BCL-2 and c-MYC in the combination group compared to single agent therapy. This study identifies S100A9 as a novel molecular target to treat AML and supports the therapeutic evaluation of tasquinimod in venetoclax-based regimens for AML patients.
© 2023. The Author(s).
Conflict of interest statement
This study was in part funded by Active Biotech.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

