Transcriptional profiles of pulmonary artery endothelial cells in pulmonary hypertension
- PMID: 38110438
- PMCID: PMC10728171
- DOI: 10.1038/s41598-023-48077-6
Transcriptional profiles of pulmonary artery endothelial cells in pulmonary hypertension
Abstract
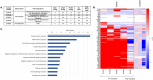
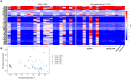
Pulmonary arterial hypertension (PAH) is characterized by endothelial cell (EC) dysfunction. There are no data from living patients to inform whether differential gene expression of pulmonary artery ECs (PAECs) can discern disease subtypes, progression and pathogenesis. We aimed to further validate our previously described method to propagate ECs from right heart catheter (RHC) balloon tips and to perform additional PAEC phenotyping. We performed bulk RNA sequencing of PAECs from RHC balloons. Using unsupervised dimensionality reduction and clustering we compared transcriptional signatures from PAH to controls and other forms of pulmonary hypertension. Select PAEC samples underwent single cell and population growth characterization and anoikis quantification. Fifty-four specimens were analyzed from 49 subjects. The transcriptome appeared stable over limited passages. Six genes involved in sex steroid signaling, metabolism, and oncogenesis were significantly upregulated in PAH subjects as compared to controls. Genes regulating BMP and Wnt signaling, oxidative stress and cellular metabolism were differentially expressed in PAH subjects. Changes in gene expression tracked with clinical events in PAH subjects with serial samples over time. Functional assays demonstrated enhanced replication competency and anoikis resistance. Our findings recapitulate fundamental biological processes of PAH and provide new evidence of a cancer-like phenotype in ECs from the central vasculature of PAH patients. This "cell biopsy" method may provide insight into patient and lung EC heterogeneity to advance precision medicine approaches in PAH.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

