Fenofibrate reduces glucose-induced barrier dysfunction in feline enteroids
- PMID: 38110453
- PMCID: PMC10728136
- DOI: 10.1038/s41598-023-49874-9
Fenofibrate reduces glucose-induced barrier dysfunction in feline enteroids
Abstract
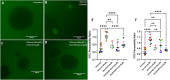
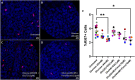
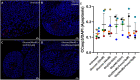
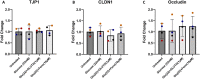
Diabetes mellitus (DM) is a common chronic metabolic disease in humans and household cats that is characterized by persistent hyperglycemia. DM is associated with dysfunction of the intestinal barrier. This barrier is comprised of an epithelial monolayer that contains a network of tight junctions that adjoin cells and regulate paracellular movement of water and solutes. The mechanisms driving DM-associated barrier dysfunction are multifaceted, and the direct effects of hyperglycemia on the epithelium are poorly understood. Preliminary data suggest that fenofibrate, An FDA-approved peroxisome proliferator-activated receptor-alpha (PPARα) agonist drug attenuates intestinal barrier dysfunction in dogs with experimentally-induced DM. We investigated the effects of hyperglycemia-like conditions and fenofibrate treatment on epithelial barrier function using feline intestinal organoids. We hypothesized that glucose treatment directly increases barrier permeability and alters tight junction morphology, and that fenofibrate administration can ameliorate these deleterious effects. We show that hyperglycemia-like conditions directly increase intestinal epithelial permeability, which is mitigated by fenofibrate. Moreover, increased permeability is caused by disruption of tight junctions, as evident by increased junctional tortuosity. Finally, we found that increased junctional tortuosity and barrier permeability in hyperglycemic conditions were associated with increased protein kinase C-α (PKCα) activity, and that fenofibrate treatment restored PKCα activity to baseline levels. We conclude that hyperglycemia directly induces barrier dysfunction by disrupting tight junction structure, a process that is mitigated by fenofibrate. We further propose that counteracting modulation of PKCα activation by increased intracellular glucose levels and fenofibrate is a key candidate regulatory pathway of tight junction structure and epithelial permeability.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kaufman FR. Type 2 diabetes mellitus in children and youth: a new epidemic. J. Pediatr. Endocrinol. Metab. 2002;15(Suppl 2):737–744. - PubMed
-
- Hospital, B.P., State of Pet Health 2016 Report, in State of Pet Health Report. 2016, Banfield Pet Hospital.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

