Augmented reality versus standard tests to assess cognition and function in early Alzheimer's disease
- PMID: 38110486
- PMCID: PMC10728213
- DOI: 10.1038/s41746-023-00978-6
Augmented reality versus standard tests to assess cognition and function in early Alzheimer's disease
Abstract
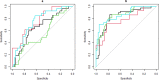
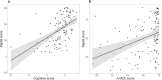
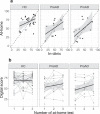
Augmented reality (AR) apps, in which the virtual and real world are combined, can recreate instrumental activities of daily living (IADL) and are therefore promising to measure cognition needed for IADL in early Alzheimer's disease (AD) both in the clinic and in the home settings. The primary aim of this study was to distinguish and classify healthy controls (HC) from participants with AD pathology in an early AD stage using an AR app. The secondary aims were to test the association of the app with clinical cognitive and functional tests and investigate the feasibility of at-home testing using AR. We furthermore investigated the test-retest reliability and potential learning effects of the task. The digital score from the AR app could significantly distinguish HC from preclinical AD (preAD) and prodromal AD (proAD), and preAD from proAD, both with in-clinic and at-home tests. For the classification of the proAD group, the digital score (AUCclinic_visit = 0.84 [0.75-0.93], AUCat_home = 0.77 [0.61-0.93]) was as good as the cognitive score (AUC = 0.85 [0.78-0.93]), while for classifying the preAD group, the digital score (AUCclinic_visit = 0.66 [0.53-0.78], AUCat_home = 0.76 [0.61-0.91]) was superior to the cognitive score (AUC = 0.55 [0.42-0.68]). In-clinic and at-home tests moderately correlated (rho = 0.57, p < 0.001). The digital score was associated with the clinical cognitive score (rho = 0.56, p < 0.001). No learning effects were found. Here we report the AR app distinguishes HC from otherwise healthy Aβ-positive individuals, both in the outpatient setting and at home, which is currently not possible with standard cognitive tests.
© 2023. The Author(s).
Conflict of interest statement
R.L.H., A.S.C. and I.T. are employees of Altoida Inc. and declare no non-financial competing interests. S.V. is an employee of Janssen Pharmaceutical NV and may hold stock options or shares in the company, but has no non-financial competing interests. D.A. has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals, Biogen, and GE Health, and served as a paid consultant for H. Lundbeck, Eisai, Heptares, Mentis Cura, and Roche Diagnostics, but declares no non-financial competing interests. S.G. declares support for this work through the Italian Ministry of Health (Ricerca Corrente). All other authors declare no financial or non-financial competing interests.
Figures



References
-
- Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. - DOI - PMC - PubMed
-
- Dubbelman MA, et al. Trajectories of decline in cognitively complex everyday activities across the Alzheimer’s disease continuum: Neuropsychology: Longitudinal cognitive assessment in early stages of AD. Alzheimers Dement. 2020;16:e044787. doi: 10.1002/alz.044787. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

