Concurrent gliomas in patients with multiple sclerosis
- PMID: 38110626
- PMCID: PMC10728097
- DOI: 10.1038/s43856-023-00381-y
Concurrent gliomas in patients with multiple sclerosis
Abstract
Background: Concurrent malignant brain tumors in patients with multiple sclerosis (MS) constitute a rare but paradigmatic phenomenon for studying neuroimmunological mechanisms from both molecular and clinical perspectives.
Methods: A multicenter cohort of 26 patients diagnosed with both primary brain tumors and multiple sclerosis was studied for disease localization, tumor treatment-related MS activity, and molecular characteristics specific for diffuse glioma in MS patients.
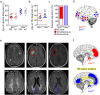
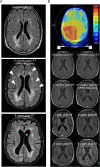
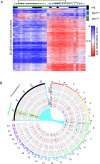
Results: MS neither predisposes nor protects from the development of gliomas. Patients with glioblastoma WHO grade 4 without isocitratdehydrogenase (IDH) mutations have a longstanding history of MS, whereas patients diagnosed with IDH-mutant astrocytoma WHO grade 2 receive multiple sclerosis diagnosis mostly at the same time or later. Concurrent MS is associated with a lesser extent of tumor resection and a worse prognosis in IDH-mutant glioma patients (PFS 32 vs. 64 months, p = 0.0206). When assessing tumor-intrinsic differences no distinct subgroup-defining methylation pattern is identified in gliomas of MS patients compared to other glioma samples. However, differential methylation of immune-related genetic loci including human leukocyte antigen locus on 6p21 and interleukin locus on 5q31 is found in MS patients vs. matched non-MS patients. In line, inflammatory disease activity increases in 42% of multiple sclerosis patients after brain tumor radiotherapy suggesting a susceptibility of multiple sclerosis brain tissue to pro-inflammatory stimuli such as ionizing radiation.
Conclusions: Concurrent low-grade gliomas should be considered in multiple sclerosis patients with slowly progressive, expansive T2/FLAIR lesions. Our findings of typically reduced extent of resection in MS patients and increased MS activity after radiation may inform future treatment decisions.
Plain language summary
Brain tumors such as gliomas can evade attacks by the immune system. In contrast, some diseases of the central nervous system such as multiple sclerosis (MS) are caused by an overactive immune system. Our study looks at a cohort of rare patients with both malignant glioma and concurrent MS and examines how each disease and their treatments affect each other. Our data suggest that even in patients with known MS, if medical imaging findings are unusual, a concurrent brain tumor should be excluded at an early stage. Radiotherapy, as is the standard of care for malignant brain tumors, may worsen the inflammatory disease activity in MS patients, which may be associated with certain genetic risk factors. Our findings may help to inform treatment of patients with brain tumors and MS.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

