Review of Adalimumab Biosimilar SB5 in Immune-Mediated Inflammatory Diseases
- PMID: 38110655
- PMCID: PMC10838831
- DOI: 10.1007/s12325-023-02737-1
Review of Adalimumab Biosimilar SB5 in Immune-Mediated Inflammatory Diseases
Abstract
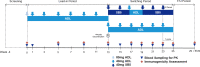
SB5 is an approved biosimilar of adalimumab, a recombinant monoclonal anti-tumor necrosis factor (TNF) antibody. The approval of SB5 was based on the comparison with reference adalimumab in analytical studies, pharmacokinetic (PK) and immunogenicity assessments, and randomized controlled trials. Efficacy data was primarily obtained in patients with rheumatoid arthritis, and extended to include additional indications such as psoriasis, Crohn's disease, or ulcerative colitis by extrapolation. Following its approval, additional post-marketing data have been collected comparing SB5 with reference adalimumab. This review summarizes the clinical data on SB5 from randomized controlled trials and provides a comprehensive overview of the available post-approval data. In "real-world" settings, SB5 was as effective as its reference product across different indications and countries, treatment persistence was well maintained throughout studies, and no new safety concerns were identified. In both controlled and "real-world" settings, switching from reference adalimumab to SB5 was not associated with altered efficacy or clinical complications. In post-approval studies, the quality of SB5 was consistent over time, independent of the batch and process changes, and the SB5 autoinjector was preferred over other autoinjectors by both healthcare professionals and patients. Taken together, these data support the use of SB5 whenever reference adalimumab is appropriate and demonstrate that switching from reference adalimumab to SB5 is feasible.
Keywords: Adalimumab; Anti-TNF; Biosimilar; Crohn’s disease; Inflammatory bowel disease; Psoriasis; Rheumatoid arthritis; SB5; Ulcerative colitis.
© 2023. The Author(s).
Conflict of interest statement
Jonathan Kay has received research support paid to UMass Chan Medical School from Aker BioMarine AS; Biogen; Galapagos NV; Novartis Pharmaceuticals Corp.; and SetPoint Medical Corp. He has received consulting fees from AbbVie Inc.; Alvotech Swiss AG; Amgen Inc.; Boehringer Ingelheim GmbH; Bristol Myers Squibb Co.; Fresenius Kabi; Novartis Pharmaceuticals Corp.; Organon LLC; Pfizer Inc.; Samsung Bioepis; Sandoz Inc.; Scipher Medicine; Teijin Pharma Ltd.; UCB Inc.; Viatris Inc.; and Yuhan Corp. He has received fees for participation in independent data safety monitoring committees from Inmagene LLC and Kolon TissueGene, Inc. He has received royalties from Wolters Kluwer NV for UpToDate. Raymond K. Cross has received consulting fees or honoraria from Abbvie, BMS, Fresenius Kabi, Fzata, Janssen, Magellan Health, Option Care, Pfizer, Samsung Bioepis, Sandoz, Sebela, and Takeda. He received fees for participation in review activities from Adiso, serves as executive committee member in the IBD education group, and as scientific co-director for CorEvitas. Steven R. Feldman has received research, speaking and/or consulting support from Eli Lilly and Company, AbbVie, Janssen, Alvotech, vTv Therapeutics, Bristol-Myers Squibb, Samsung, Pfizer, Boehringer Ingelheim, Amgen, Dermavant, Arcutis, Novartis, UCB, Helsinn, Sun Pharma, Almirall, Galderma, Mylan, Forte, Arena, Biocon, Accordant, Argenx, Sanofi, Regeneron, the National Biological Corporation, Caremark, Teladoc, BMS, Ono, and Micreos. He is a founder and part owner of Causa Research and holds stock in Sensal Health. Younjin Park is an employee of Samsung Bioepis. Stephen B. Hanauer has received financial research, speaking, review, and/or consulting support from Abbvie, Allergan, Amgen, Arena, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cosmos, Catalys Pacific, Covance, Genentech, GSK, Janssen, Lilly, Merck, Novartis, Pfizer, Progenity, Prometheus, Receptos, Salix, Samsung Bioepis, Seres Therapeutics, Sorriso, Takeda, TLL Pharma, UCB Pharma, VHsquared, Gossamer, Protagonist.
Figures


References
-
- FDA. Prescribing information—HUMIRA (Adalimumab). 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125057s417lbl.pdf. Accessed 30 Mar 2023.
-
- FDA. Purple book—database of licensed biological products. 2023. https://purplebooksearch.fda.gov/. Accessed 30 Mar 2023.
-
- FDA. What is a biosimilar? https://www.fda.gov/media/108905/download. Accessed 30 Mar 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

