Commensal production of a broad-spectrum and short-lived antimicrobial peptide polyene eliminates nasal Staphylococcus aureus
- PMID: 38110697
- PMCID: PMC11310079
- DOI: 10.1038/s41564-023-01544-2
Commensal production of a broad-spectrum and short-lived antimicrobial peptide polyene eliminates nasal Staphylococcus aureus
Erratum in
-
Author Correction: Commensal production of a broad-spectrum and short-lived antimicrobial peptide polyene eliminates nasal Staphylococcus aureus.Nat Microbiol. 2024 Dec;9(12):3393. doi: 10.1038/s41564-024-01798-4. Nat Microbiol. 2024. PMID: 39117907 Free PMC article. No abstract available.
Abstract
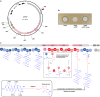
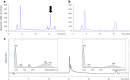
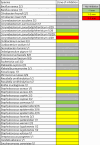
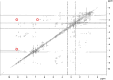
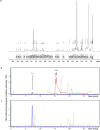
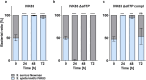
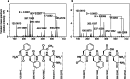
Antagonistic bacterial interactions often rely on antimicrobial bacteriocins, which attack only a narrow range of target bacteria. However, antimicrobials with broader activity may be advantageous. Here we identify an antimicrobial called epifadin, which is produced by nasal Staphylococcus epidermidis IVK83. It has an unprecedented architecture consisting of a non-ribosomally synthesized peptide, a polyketide component and a terminal modified amino acid moiety. Epifadin combines a wide antimicrobial target spectrum with a short life span of only a few hours. It is highly unstable under in vivo-like conditions, potentially as a means to limit collateral damage of bacterial mutualists. However, Staphylococcus aureus is eliminated by epifadin-producing S. epidermidis during co-cultivation in vitro and in vivo, indicating that epifadin-producing commensals could help prevent nasal S. aureus carriage. These insights into a microbiome-derived, previously unknown antimicrobial compound class suggest that limiting the half-life of an antimicrobial may help to balance its beneficial and detrimental activities.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





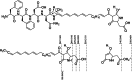


References
MeSH terms
Substances
Grants and funding
- Cluster of Excellence EXC2124 Controlling Microbes to Fight Infection; project ID 390838134/Deutsche Forschungsgemeinschaft (German Research Foundation)
- ZUK 63/Deutsche Forschungsgemeinschaft (German Research Foundation)
- TRR261 (project ID 398967434), GRK1708/Deutsche Forschungsgemeinschaft (German Research Foundation)
- TRR261 (project ID 398967434),GRK1708/Deutsche Forschungsgemeinschaft (German Research Foundation)
- TRR261 (project ID 398967434), TRR156 (project ID 246807620), GRK1708/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

