Rotaxane-Functionalized Dyes for Charge-Rectification in p-Type Photoelectrochemical Devices
- PMID: 38110821
- PMCID: PMC10916627
- DOI: 10.1002/advs.202306032
Rotaxane-Functionalized Dyes for Charge-Rectification in p-Type Photoelectrochemical Devices
Abstract
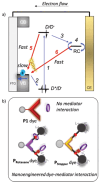
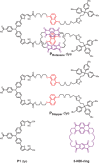
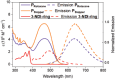
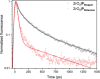
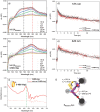
A supramolecular photovoltaic strategy is applied to enhance power conversion efficiencies (PCE) of photoelectrochemical devices by suppressing electron-hole recombination after photoinduced electron transfer (PET). Here, the author exploit supramolecular localization of the redox mediator-in close proximity to the dye-through a rotaxane topology, reducing electron-hole recombination in p-type dye-sensitized solar cells (p-DSSCs). Dye PRotaxane features 1,5-dioxynaphthalene recognition sites (DNP-arms) with a mechanically-interlocked macrocyclic redox mediator naphthalene diimide macrocycle (3-NDI-ring), stoppering synthetically via click chemistry. The control molecule PStopper has stoppered DNP-arms, preventing rotaxane formation with the 3-NDI-ring. Transient absorption and time-resolved fluorescence spectroscopy studies show ultrafast (211 ± 7 fs and 2.92 ± 0.05 ps) PET from the dye-moiety of PRotaxane to its mechanically interlocked 3-NDI-ring-acceptor, slowing down the electron-hole recombination on NiO surfaces compared to the analogue . p-DSSCs employing PRotaxane (PCE = 0.07%) demonstrate a 30% PCE increase compared to PStopper (PCE = 0.05%) devices, combining enhancements in both open-circuit voltages (VOC = 0.43 vs 0.36 V) and short-circuit photocurrent density (JSC = -0.39 vs -0.34 mA cm-2 ). Electrochemical impedance spectroscopy shows that PRotaxane devices exhibit hole lifetimes (τh ) approaching 1 s, a 16-fold improvement compared to traditional I- /I3 - -based systems (τh = 50 ms), demonstrating the benefits obtained upon nanoengineering of interfacial dye-regeneration at the photocathode.
Keywords: femtosecond transient absorption; interfacial photoelectrochemistry; p-type dye-sensitized solar cell; rotaxanes; supramolecular electronics.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Arunkumar E., Fu N., Smith B. D., Chemistry 2006, 12, 4684. - PubMed
-
- Goto Y., Hisatomi T., Wang Q., Higashi T., Ishikiriyama K., Maeda T., Sakata Y., Okunaka S., Tokudome H., Katayama M., Akiyama S., Nishiyama H., Inoue Y., Takewaki T., Setoyama T., Minegishi T., Takata T., Yamada T., Domen K., Joule 2018, 2, 509.
-
- Li H., Fahrenbach A. C., Coskun A., Zhu Z., Barin G., Zhao Y.‐L., Botros Y. Y., Sauvage J.‐P., Stoddart J. F., Angew. Chem., Int. Ed. 2011, 50, 6782. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
