Engrailed-1 Promotes Pancreatic Cancer Metastasis
- PMID: 38110836
- PMCID: PMC10853725
- DOI: 10.1002/advs.202308537
Engrailed-1 Promotes Pancreatic Cancer Metastasis
Abstract
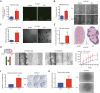
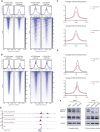
Engrailed-1 (EN1) is a critical homeodomain transcription factor (TF) required for neuronal survival, and EN1 expression has been shown to promote aggressive forms of triple negative breast cancer. Here, it is reported that EN1 is aberrantly expressed in a subset of pancreatic ductal adenocarcinoma (PDA) patients with poor outcomes. EN1 predominantly repressed its target genes through direct binding to gene enhancers and promoters, implicating roles in the activation of MAPK pathways and the acquisition of mesenchymal cell properties. Gain- and loss-of-function experiments demonstrated that EN1 promoted PDA transformation and metastasis in vitro and in vivo. The findings nominate the targeting of EN1 and downstream pathways in aggressive PDA.
Keywords: ERK signaling; Engrailed-1; apoptosis; cancer progression; cancer therapeutics; developmental transcription factor; epigenetic reprogramming; metastasis; pancreatic ductal adenocarcinoma.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- U01CA224013/GF/NIH HHS/United States
- P30 CA093373/CA/NCI NIH HHS/United States
- R01 CA279560/CA/NCI NIH HHS/United States
- R50 CA211506/CA/NCI NIH HHS/United States
- U01 CA224013/CA/NCI NIH HHS/United States
- T32 ES007059/ES/NIEHS NIH HHS/United States
- R01 CA229699/CA/NCI NIH HHS/United States
- K22 CA226037/CA/NCI NIH HHS/United States
- P01 CA013106/CA/NCI NIH HHS/United States
- R21 CA252162/CA/NCI NIH HHS/United States
- R37 CA249007/CA/NCI NIH HHS/United States
- R01 CA188134/CA/NCI NIH HHS/United States
- R01 HL165135/HL/NHLBI NIH HHS/United States
- R01CA188134/GF/NIH HHS/United States
- T32ES007059/ES/NIEHS NIH HHS/United States
- P50 CA127297/CA/NCI NIH HHS/United States
- R50 CA211462/CA/NCI NIH HHS/United States
- U01CA210240/GF/NIH HHS/United States
- P30 CA036727/CA/NCI NIH HHS/United States
- P30CA45508/GF/NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- R50CA211462/CA/NCI NIH HHS/United States
- U01 CA210240/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
