Microbiological evaluation of conjunctival anopthalmic flora after using digital 3D-printed ocular prosthesis compared to conventional one: a randomized clinical trial
- PMID: 38110937
- PMCID: PMC10729395
- DOI: 10.1186/s12903-023-03746-w
Microbiological evaluation of conjunctival anopthalmic flora after using digital 3D-printed ocular prosthesis compared to conventional one: a randomized clinical trial
Abstract
Background: This study aims to assess the influence of using 3D-printed acrylic resin versus conventional Poly-methyl methacrylate (PMMA) for fabricating ocular prostheses on the biofilm and microbial flora of anophthalmic socket.
Methods: A randomized controlled trial was designed as a parallel group study. Participants were allocated randomly into two groups: the control group, which received conventionally fabricated ocular prostheses (CG, n = 11), and the test group, which received digitally 3D-printed ocular prostheses (DG, n = 11). Microbiological analysis was conducted before prosthesis insertion and three months after using the ocular prosthesis. Swab samples were inoculated on blood agar, MacConkey's agar, and Sabouraud's dextrose agar (SDA) for isolating Gram-positive, Gram-negative, and fungal organisms, respectively. Subsequently, the plates were incubated at 37 degrees Celsius for 48 h. Additionally, a validated questionnaire was used for subjective clinical evaluation, including parameters such as comfort level, socket discharge, lacrimation, and frequency of lubrication for each ocular prosthesis patient in both groups.
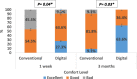
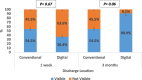
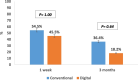
Results: Test group (DG, n = 11) exhibited a positive, though statistically insignificant, difference (p > 0.001) in microbial growth when compared to the control group (CG, n = 11). A statistically significant difference was observed in comfort levels between the two groups, with more comfort level within group II (test group) patients. While parameters such as discharge amount, discharge location, lacrimation and lubrication frequency displayed statistically insignificant differences between the two groups, all parameters showed improved results after three months of prosthesis use.
Conclusions: The choice of ocular prosthesis fabrication technique did not yield a statistically significant difference in anophthalmic flora. However, the 3D-printed acrylic resin, as an artificial eye material, displayed potential advantages in reducing the colonization of opportunistic pathogens. All subjective clinical evaluation parameters exhibited enhanced outcomes after three months of prosthesis use, emphasizing the need for an adaptation period during which patients complains are alleviated. In comparison with PMMA, 3D-printed acrylic resin showcased a certain degree of anti-colonization ability against pathogenic bacteria, along with a significant level of patient comfort, suggesting its potential as a promising material for ocular prostheses.
Trial registration: This parallel double-blinded RCT has been registered at ClinicalTrials.gov with identification number: NCT05584865, 18/10/2022.
Keywords: 3D printing; Anophthalmic flora; Computer aided; Ocular prosthesis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Miller SD, Smith RE, Dippe DW, Lacey DR, Abel M. Bacteriology of the socket in patients with prostheses. Can J Ophthalmol. 1976;11:126–9. - PubMed
-
- Patillon JC, Rousse C, Gauthier C, Guyot J, Barbier A, Royer J, et al. Bacterial flora of the conjunctiva in enucleated subjects. Bull Soc Ophtalmol Fr. 1978;78:781–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

