Targeting CB2R in astrocytes for Parkinson's disease therapy: unraveling the Foxg1-mediated neuroprotective mechanism through autophagy-mediated NLRP3 degradation
- PMID: 38110963
- PMCID: PMC10729372
- DOI: 10.1186/s12974-023-02989-2
Targeting CB2R in astrocytes for Parkinson's disease therapy: unraveling the Foxg1-mediated neuroprotective mechanism through autophagy-mediated NLRP3 degradation
Abstract
Background: Inflammasomes in astrocytes have been shown to play a crucial role in the pathogenesis of neurodegenerative diseases such as Parkinson's disease (PD) and Alzheimer's disease (AD). Cannabinoid Receptor 2(CB2R), a G protein-coupled receptor (GPCR), is considered a promising therapeutic target in inflammation-related disorders. This study aims to explore the role of CB2R in regulating NOD-like receptor family pyrin domain containing 3 (NLRP3)-mediated neuroinflammation in astrocytes.
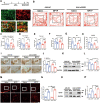
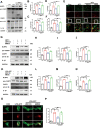
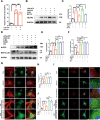
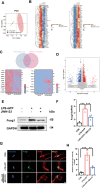
Methods: In an in vivo animal model, specific targeting of astrocytic CB2R was achieved by injecting CB2R-specific adenovirus (or fork head box g1(foxg1) adenovirus) to knock down CB2R or administering CB2R agonists, inhibitors, etc., in the substantia nigra pars compacta (SNc) of mice. A PD mouse model was established using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induction. Animal behavioral tests, western blot, immunofluorescence, and other experiments were performed to assess the loss of midbrain tyrosine hydroxylase (TH) neurons, activation of astrocytes, and activation of the NLRP3 pathway. Primary astrocytes were cultured in vitro, and NLRP3 inflammasomes were activated using 1-methyl-4-phenylpyridinium (MPP+) or lipopolysaccharide (LPS) and adenosine triphosphate (ATP). Western blot and ELISA experiments were conducted to assess the release of inflammatory factors. Transcriptomic sequencing and CUT&RUN techniques were employed to study the CB2R regulation of the foxg1 binding site on the autophagy molecule microtubule-associated protein 1 light chain 3 beta (MAP1LC3B).
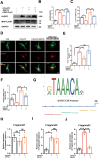
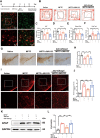
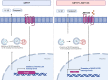
Results: Astrocytic CB2R knockdown impaired the motor abilities of MPTP-induced mice, exacerbated the loss of TH neurons, and induced activation of the NLRP3/Caspase-1/interleukin 1 (IL-1β) pathway. Activation of CB2R significantly alleviated motor impairments in mice while reducing NLRP3 deposition on astrocytes. In vitro cell experiments showed that CB2R activation attenuated the activation of the NLRP3/Caspase-1/IL-1β pathway induced by LPS + ATP or MPP+. Additionally, it inhibited the binding of foxg1 to MAP1LC3B, increased astrocytic autophagy levels, and facilitated NLRP3 degradation through the autophagy-lysosome pathway.
Conclusion: Activation of CB2R on astrocytes effectively mitigates NLRP3-mediated neuroinflammation and ameliorates the disease characteristics of PD in mice. CB2R represents a potential therapeutic target for treating PD.
Keywords: Autophagy; CB2R; NLRP3; Parkinson disease.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

