Phase separations in oncogenesis, tumor progressions and metastasis: a glance from hallmarks of cancer
- PMID: 38110976
- PMCID: PMC10726551
- DOI: 10.1186/s13045-023-01522-5
Phase separations in oncogenesis, tumor progressions and metastasis: a glance from hallmarks of cancer
Abstract
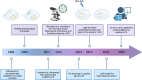
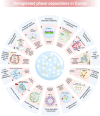
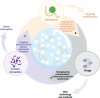
Liquid-liquid phase separation (LLPS) is a novel principle for interpreting precise spatiotemporal coordination in living cells through biomolecular condensate (BMC) formation via dynamic aggregation. LLPS changes individual molecules into membrane-free, droplet-like BMCs with specific functions, which coordinate various cellular activities. The formation and regulation of LLPS are closely associated with oncogenesis, tumor progressions and metastasis, the specific roles and mechanisms of LLPS in tumors still need to be further investigated at present. In this review, we comprehensively summarize the conditions of LLPS and identify mechanisms involved in abnormal LLPS in cancer processes, including tumor growth, metastasis, and angiogenesis from the perspective of cancer hallmarks. We have also reviewed the clinical applications of LLPS in oncologic areas. This systematic summary of dysregulated LLPS from the different dimensions of cancer hallmarks will build a bridge for determining its specific functions to further guide basic research, finding strategies to intervene in LLPS, and developing relevant therapeutic approaches.
Keywords: Biomolecular condensate; Cancer; Liquid–liquid phase separation; Membrane-less organelle; Novel therapeutics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
- 82203860/National Natural Science Foundation of China
- 82072916/National Natural Science Foundation of China
- 82325042/National Outstanding Youth Science Fund Project of National Natural Science Foundation of China
- 2023-05-50/Major Innovation Program of Shanghai Education Commission
- 2023YFC3404100/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Medical

