CD115+ monocytes protect microbially experienced mice against E. coli-induced sepsis
- PMID: 38111515
- PMCID: PMC10727454
- DOI: 10.1016/j.celrep.2023.113345
CD115+ monocytes protect microbially experienced mice against E. coli-induced sepsis
Abstract
Uropathogenic E. coli (UPEC) is a primary organism responsible for urinary tract infections and a common cause of sepsis. Microbially experienced laboratory mice, generated by cohousing with pet store mice, exhibit increased morbidity and mortality to polymicrobial sepsis or lipopolysaccharide challenge. By contrast, cohoused mice display significant resistance, compared with specific pathogen-free mice, to a monomicrobial sepsis model using UPEC. CD115+ monocytes mediate protection in the cohoused mice, as depletion of these cells leads to increased mortality and UPEC pathogen burden. Further study of the cohoused mice reveals increased TNF-α production by monocytes, a skewing toward Ly6ChiCD115+ "classical" monocytes, and enhanced egress of Ly6ChiCD115+ monocytes from the bone marrow. Analysis of cohoused bone marrow also finds increased frequency and number of myeloid multipotent progenitor cells. These results show that a history of microbial exposure impacts innate immunity in mice, which can have important implications for the preclinical study of sepsis.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures
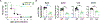
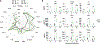
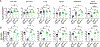

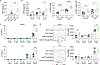


Similar articles
-
Protocol for inducing monomicrobial sepsis in mice with uropathogenic E. coli.STAR Protoc. 2024 Sep 20;5(3):103206. doi: 10.1016/j.xpro.2024.103206. Epub 2024 Jul 27. STAR Protoc. 2024. PMID: 39068653 Free PMC article.
-
Atg16L1 deficiency confers protection from uropathogenic Escherichia coli infection in vivo.Proc Natl Acad Sci U S A. 2012 Jul 3;109(27):11008-13. doi: 10.1073/pnas.1203952109. Epub 2012 Jun 19. Proc Natl Acad Sci U S A. 2012. PMID: 22715292 Free PMC article.
-
Group 3 Innate Lymphoid Cells Protect the Host from the Uropathogenic Escherichia coli Infection in the Bladder.Adv Sci (Weinh). 2022 Feb;9(6):e2103303. doi: 10.1002/advs.202103303. Epub 2022 Jan 12. Adv Sci (Weinh). 2022. PMID: 35018740 Free PMC article.
-
Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection.Curr Opin Microbiol. 2013 Feb;16(1):100-7. doi: 10.1016/j.mib.2013.01.005. Epub 2013 Feb 8. Curr Opin Microbiol. 2013. PMID: 23403118 Review.
-
A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections.Microb Pathog. 2020 Jul;144:104196. doi: 10.1016/j.micpath.2020.104196. Epub 2020 Apr 10. Microb Pathog. 2020. PMID: 32283258
Cited by
-
Integrating natural commensals and pathogens into preclinical mouse models.Nat Rev Immunol. 2025 May;25(5):385-397. doi: 10.1038/s41577-024-01108-3. Epub 2024 Nov 19. Nat Rev Immunol. 2025. PMID: 39562646 Free PMC article. Review.
-
Florfenicol-induced dysbiosis impairs intestinal homeostasis and host immune system in laying hens.J Anim Sci Biotechnol. 2025 Apr 14;16(1):56. doi: 10.1186/s40104-025-01186-w. J Anim Sci Biotechnol. 2025. PMID: 40223090 Free PMC article.
-
Physiological microbial exposure normalizes memory T cell surveillance of the brain and modifies host seizure outcomes.Nat Immunol. 2025 Jul;26(7):1087-1098. doi: 10.1038/s41590-025-02174-y. Epub 2025 Jun 13. Nat Immunol. 2025. PMID: 40514419
-
Enhancing acute inflammatory and sepsis treatment: superiority of membrane receptor blockade.Front Immunol. 2024 Jul 16;15:1424768. doi: 10.3389/fimmu.2024.1424768. eCollection 2024. Front Immunol. 2024. PMID: 39081318 Free PMC article. Review.
-
Mouse Models of Sepsis.Curr Protoc. 2024 Mar;4(3):e997. doi: 10.1002/cpz1.997. Curr Protoc. 2024. PMID: 38439603 Free PMC article.
References
-
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. (2020). Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395, 200–211. 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

