Host serine protease ACOT2 assists DENV proliferation by hydrolyzing viral polyproteins
- PMID: 38112462
- PMCID: PMC10804956
- DOI: 10.1128/msystems.00973-23
Host serine protease ACOT2 assists DENV proliferation by hydrolyzing viral polyproteins
Abstract
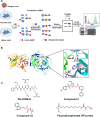
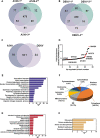
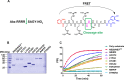
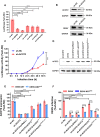
Dengue fever is a mosquito-borne tropical disease caused by the dengue virus (DENV). The replication of DENV relies on the processing of its genome-encoded polyprotein by both viral protease NS3 (NS3pro) and host proteases. However, the impact of host proteases on DENV proliferation is not well understood. In this study, we utilized fluorophosphonate-based probes (FPs) to investigate the up-regulation of host serine proteases during DENV infection in detail. Among the identified proteases, acyl-CoA thioesterase 2 (ACOT2), an enzyme that hydrolyzes acyl-CoA molecules to generate fatty acids and free CoA, exhibited cleavage activity against DENV polypeptide substrates. Enzymatic assays and virological experiments confirmed that ACOT2 contributes to DENV propagation during the replication stage by cleaving the viral polyprotein. Docking models provided insights into the binding pocket of viral polypeptides and the catalytic mechanism of ACOT2. Notably, this study is the first to demonstrate that ACOT2 functions as a serine protease to hydrolyze protein substrates. These findings offer novel insights into DENV infection, host response, as well as the potential development of innovative antiviral strategies.IMPORTANCEDENV, one of the major pathogens of Dengue fever, remains a significant public health concern in tropical and subtropical regions worldwide. How DENV efficiently hijacks the host and accesses its life cycle with delicate interaction remains to be elucidated. Here, we deconvoluted that the host protease ACOT2 assists the DENV replication and characterized the ACOT2 as a serine protease involved in the hydrolysis of the DENV polypeptide substrate. Our results not only further the understanding of the DENV life cycle but also provide a possibility for the usage of activity-based proteomics to reveal host-virus interactions.
Keywords: ABPP; ACOT2; DENV; serine protease; virus replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dengue Virus NS2B/NS3 Protease Inhibitors Exploiting the Prime Side.J Virol. 2017 Apr 28;91(10):e00045-17. doi: 10.1128/JVI.00045-17. Print 2017 May 15. J Virol. 2017. PMID: 28298600 Free PMC article.
-
Dengue Protease Substrate Recognition: Binding of the Prime Side.ACS Infect Dis. 2016 Oct 14;2(10):734-743. doi: 10.1021/acsinfecdis.6b00131. Epub 2016 Sep 22. ACS Infect Dis. 2016. PMID: 27657335 Free PMC article.
-
Dengue virus non-structural protein 3 inhibits mitochondrial respiration by impairing complex I function.mSphere. 2024 Jul 30;9(7):e0040624. doi: 10.1128/msphere.00406-24. Epub 2024 Jul 9. mSphere. 2024. PMID: 38980068 Free PMC article.
-
Recent advances in natural products as potential inhibitors of dengue virus with a special emphasis on NS2b/NS3 protease.Phytochemistry. 2022 Oct;202:113362. doi: 10.1016/j.phytochem.2022.113362. Epub 2022 Aug 7. Phytochemistry. 2022. PMID: 35948138 Review.
-
Exploiting Dengue Virus Protease as a Therapeutic Target: Current Status, Challenges and Future Avenues.Curr Med Chem. 2021;28(37):7767-7802. doi: 10.2174/0929867328666210629152929. Curr Med Chem. 2021. PMID: 34212826 Review.
Cited by
-
Exploiting host kinases to combat dengue virus infection and disease.Antiviral Res. 2025 Sep;241:106172. doi: 10.1016/j.antiviral.2025.106172. Epub 2025 May 8. Antiviral Res. 2025. PMID: 40348023 Review.
-
Dengue virus and lipid metabolism: unravelling the interplay for future therapeutic approaches.Emerg Microbes Infect. 2025 Dec;14(1):2477647. doi: 10.1080/22221751.2025.2477647. Epub 2025 Apr 9. Emerg Microbes Infect. 2025. PMID: 40059731 Free PMC article. Review.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060 - DOI - PMC - PubMed
-
- Feitosa-Suntheimer F, Zhu Z, Mameli E, Dayama G, Gold AS, Broos-Caldwell A, Troupin A, Rippee-Brooks M, Corley RB, Lau NC, Colpitts TM, Londoño-Renteria B. 2022. Dengue virus-2 infection affects fecundity and elicits specific transcriptional changes in the ovaries of Aedes aegypti mosquitoes. Front Microbiol 13:886787. doi:10.3389/fmicb.2022.886787 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2020YFA0908500/Ministry of Science and Technology of the People's Republic of China (MOST)
- 31971127/MOST | National Natural Science Foundation of China (NSFC)
- 2021YFF1200700/Ministry of Science and Technology of the People's Republic of China (MOST)
- 81801998/MOST | National Natural Science Foundation of China (NSFC)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

