Swiss digital pathology recommendations: results from a Delphi process conducted by the Swiss Digital Pathology Consortium of the Swiss Society of Pathology
- PMID: 38112792
- PMCID: PMC11271322
- DOI: 10.1007/s00428-023-03712-5
Swiss digital pathology recommendations: results from a Delphi process conducted by the Swiss Digital Pathology Consortium of the Swiss Society of Pathology
Abstract
Integration of digital pathology (DP) into clinical diagnostic workflows is increasingly receiving attention as new hardware and software become available. To facilitate the adoption of DP, the Swiss Digital Pathology Consortium (SDiPath) organized a Delphi process to produce a series of recommendations for DP integration within Swiss clinical environments. This process saw the creation of 4 working groups, focusing on the various components of a DP system (1) scanners, quality assurance and validation of scans, (2) integration of Whole Slide Image (WSI)-scanners and DP systems into the Pathology Laboratory Information System, (3) digital workflow-compliance with general quality guidelines, and (4) image analysis (IA)/artificial intelligence (AI), with topic experts for each recruited for discussion and statement generation. The work product of the Delphi process is 83 consensus statements presented here, forming the basis for "SDiPath Recommendations for Digital Pathology". They represent an up-to-date resource for national and international hospitals, researchers, device manufacturers, algorithm developers, and all supporting fields, with the intent of providing expectations and best practices to help ensure safe and efficient DP usage.
Keywords: DP usage; Delphi process; Digital pathology; Integration; Swiss Digital Pathology Consortium.
© 2023. The Author(s).
Conflict of interest statement
Dr. Janowczyk provides consulting for Merck, Lunaphore, and Roche, the latter of which he also has a sponsored research agreement. Dr. Koelzer has acted as an invited speaker for Sharing Progress in Cancer Care (SPCC) and Indica Labs and holds sponsored research agreements with Roche and IAG. Mr. Kupferschmid is employed by Basys Data GmbH.
Figures
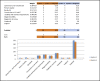
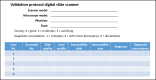
References
-
- Hanna MG, Reuter VE, Samboy J, England C, Corsale L, Fine SW, Agaram NP, Stamelos E, Yagi Y, Hameed M, Klimstra DS, Sirintrapun SJ (2019) Implementation of digital pathology offers clinical and operational increase in efficiency and cost savings. Arch Pathol Lab Med 143(12):1545–1555. 10.5858/arpa.2018-0514-OA 10.5858/arpa.2018-0514-OA - DOI - PMC - PubMed
-
- Peyster EG, Janowczyk A, Swamidoss A, Kethireddy S, Feldman MD, Margulies KB (2022) Computational analysis of routine biopsies improves diagnosis and prediction of cardiac allograft vasculopathy. Circulation 145(21):1563–1577. 10.1161/CIRCULATIONAHA.121.058459 10.1161/CIRCULATIONAHA.121.058459 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

