A dicer-related helicase opposes the age-related pathology from SKN-1 activation in ASI neurons
- PMID: 38113255
- PMCID: PMC10756303
- DOI: 10.1073/pnas.2308565120
A dicer-related helicase opposes the age-related pathology from SKN-1 activation in ASI neurons
Abstract
Coordination of cellular responses to stress is essential for health across the lifespan. The transcription factor SKN-1 is an essential homeostat that mediates survival in stress-inducing environments and cellular dysfunction, but constitutive activation of SKN-1 drives premature aging thus revealing the importance of turning off cytoprotective pathways. Here, we identify how SKN-1 activation in two ciliated ASI neurons in Caenorhabditis elegans results in an increase in organismal transcriptional capacity that drives pleiotropic outcomes in peripheral tissues. An increase in the expression of established SKN-1 stress response and lipid metabolism gene classes of RNA in the ASI neurons, in addition to the increased expression of several classes of noncoding RNA, define a molecular signature of animals with constitutive SKN-1 activation and diminished healthspan. We reveal neddylation as a unique regulator of the SKN-1 homeostat that mediates SKN-1 abundance within intestinal cells. Moreover, RNAi-independent activity of the dicer-related DExD/H-box helicase, drh-1, in the intestine, can oppose the effects of aberrant SKN-1 transcriptional activation and delays age-dependent decline in health. Taken together, our results uncover a cell nonautonomous circuit to maintain organism-level homeostasis in response to excessive SKN-1 transcriptional activity in the sensory nervous system.
Keywords: ASI neurons; C. elegans; aging; cell nonautonomous signaling; transcriptional capacity.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
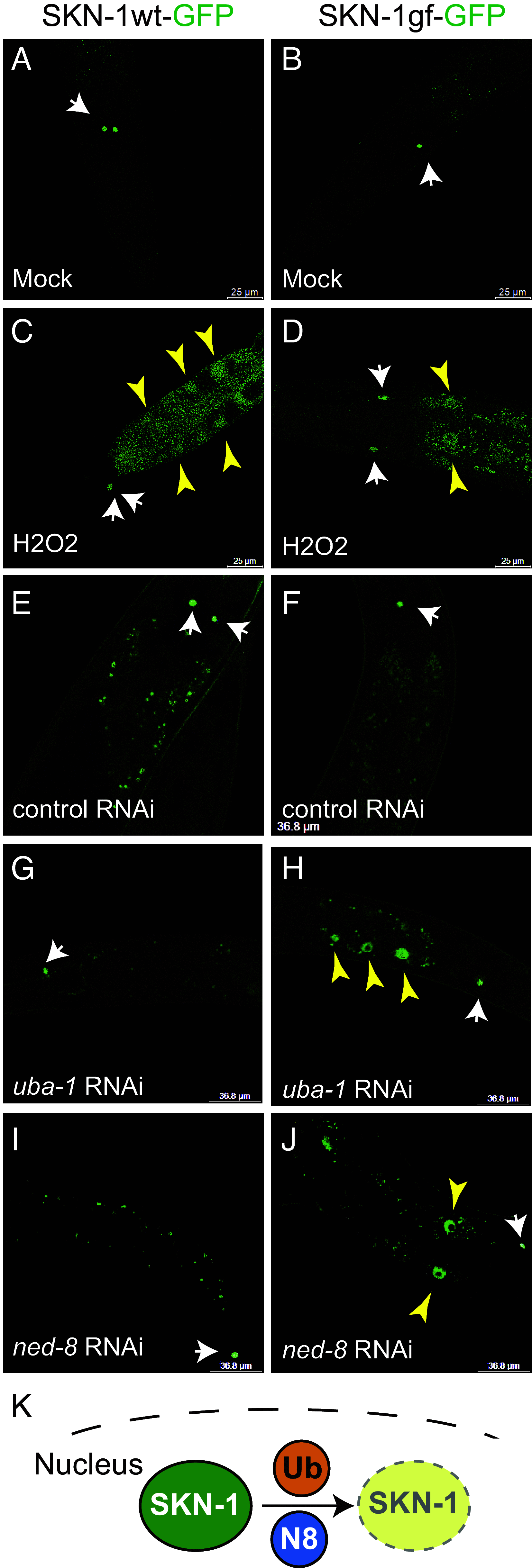


Update of
-
A dicer-related helicase opposes the age-related pathology from SKN-1 activation in ASI neurons.bioRxiv [Preprint]. 2023 Oct 2:2023.10.01.560409. doi: 10.1101/2023.10.01.560409. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2308565120. doi: 10.1073/pnas.2308565120. PMID: 37873147 Free PMC article. Updated. Preprint.
References
-
- Motohashi H., Yamamoto M., Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 10, 549–557 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

