Mathematical modeling of intratumoral immunotherapy yields strategies to improve the treatment outcomes
- PMID: 38113269
- PMCID: PMC10763956
- DOI: 10.1371/journal.pcbi.1011740
Mathematical modeling of intratumoral immunotherapy yields strategies to improve the treatment outcomes
Abstract
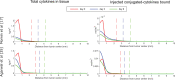
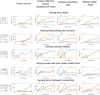
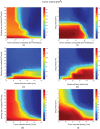
Intratumoral injection of immunotherapy aims to maximize its activity within the tumor. However, cytokines are cleared via tumor vessels and escape from the tumor periphery into the host-tissue, reducing efficacy and causing toxicity. Thus, understanding the determinants of the tumor and immune response to intratumoral immunotherapy should lead to better treatment outcomes. In this study, we developed a mechanistic mathematical model to determine the efficacy of intratumorally-injected conjugated-cytokines, accounting for properties of the tumor microenvironment and the conjugated-cytokines. The model explicitly incorporates i) the tumor vascular density and permeability and the tumor hydraulic conductivity, ii) conjugated-cytokines size and binding affinity as well as their clearance via the blood vessels and the surrounding tissue, and iii) immune cells-cancer cells interactions. Model simulations show how the properties of the tumor and of the conjugated-cytokines determine treatment outcomes and how selection of proper parameters can optimize therapy. A high tumor tissue hydraulic permeability allows for the uniform distribution of the cytokines into the tumor, whereas uniform tumor perfusion is required for sufficient access and activation of immune cells. The permeability of the tumor vessels affects the blood clearance of the cytokines and optimal values depend on the size of the conjugates. A size >5 nm in radius was found to be optimal, whereas the binding of conjugates should be high enough to prevent clearance from the tumor into the surrounding tissue. In conclusion, development of strategies to improve vessel perfusion and tissue hydraulic conductivity by reprogramming the microenvironment along with optimal design of conjugated-cytokines can enhance intratumoral immunotherapy.
Copyright: © 2023 Harkos et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
We have read the journal’s policy and the authors of this manuscript have the following competing interests: R.K.J. received consultant fees from Cur, Elpis, Innocoll, Merck, SPARC, SynDevRx; owns equity in Accurius, Enlight, SynDevRx; and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund; and received grants from Boehringer Ingelheim and Sanofi. Neither any reagent nor any funding from these organizations was used in this study.
Figures



Similar articles
-
Investigating the synergistic effects of immunotherapy and normalization treatment in modulating tumor microenvironment and enhancing treatment efficacy.J Theor Biol. 2024 Apr 21;583:111768. doi: 10.1016/j.jtbi.2024.111768. Epub 2024 Feb 23. J Theor Biol. 2024. PMID: 38401748
-
Combining two strategies to improve perfusion and drug delivery in solid tumors.Proc Natl Acad Sci U S A. 2013 Nov 12;110(46):18632-7. doi: 10.1073/pnas.1318415110. Epub 2013 Oct 28. Proc Natl Acad Sci U S A. 2013. PMID: 24167277 Free PMC article.
-
Prediction of anti-CD25 and 5-FU treatments efficacy for pancreatic cancer using a mathematical model.BMC Cancer. 2021 Nov 15;21(1):1226. doi: 10.1186/s12885-021-08770-z. BMC Cancer. 2021. PMID: 34781899 Free PMC article.
-
Harnessing Metabolic Reprogramming to Improve Cancer Immunotherapy.Int J Mol Sci. 2021 Sep 24;22(19):10268. doi: 10.3390/ijms221910268. Int J Mol Sci. 2021. PMID: 34638609 Free PMC article. Review.
-
Targeting the tumor microenvironment to enhance antitumor immune responses.Oncotarget. 2015 Jan 30;6(3):1359-81. doi: 10.18632/oncotarget.3204. Oncotarget. 2015. PMID: 25682197 Free PMC article. Review.
Cited by
-
Mathematical Modeling of Salmonella Cancer Therapies Demonstrates the Necessity of Both Bacterial Cytotoxicity and Immune Activation.Bioengineering (Basel). 2025 Jul 10;12(7):751. doi: 10.3390/bioengineering12070751. Bioengineering (Basel). 2025. PMID: 40722443 Free PMC article.
-
Synergistic mechanotherapy and sonopermeation guided by mathematical modeling for solid tumor treatment.Front Drug Deliv. 2025 Jun 24;5:1549098. doi: 10.3389/fddev.2025.1549098. eCollection 2025. Front Drug Deliv. 2025. PMID: 40837706 Free PMC article.
-
Challenges and opportunities for the next generation of computational tumor models.PLoS Biol. 2025 Jul 23;23(7):e3003269. doi: 10.1371/journal.pbio.3003269. eCollection 2025 Jul. PLoS Biol. 2025. PMID: 40700394 Free PMC article.
-
Multi-scale computational modeling towards efficacy in radiopharmaceutical therapies while minimizing side effects: Modeling of amino acid infusion.PLoS Comput Biol. 2025 Jul 16;21(7):e1013247. doi: 10.1371/journal.pcbi.1013247. eCollection 2025 Jul. PLoS Comput Biol. 2025. PMID: 40668873 Free PMC article.
-
Using mathematical modelling and AI to improve delivery and efficacy of therapies in cancer.Nat Rev Cancer. 2025 May;25(5):324-340. doi: 10.1038/s41568-025-00796-w. Epub 2025 Feb 19. Nat Rev Cancer. 2025. PMID: 39972158 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

