Tetrahedral Clusters Stabilized by Alloying
- PMID: 38113287
- PMCID: PMC10788904
- DOI: 10.1021/acs.jpca.3c06033
Tetrahedral Clusters Stabilized by Alloying
Abstract
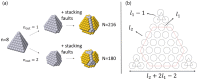
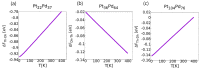
A family of nanoclusters of tetrahedral symmetry is proposed. These clusters consist of symmetrically truncated tetrahedra with additional hexagonal islands on the four facets of the starting tetrahedron. The islands are placed in stacking fault positions. The geometric magic numbers of these clusters are derived. Global optimization searches within an atomistic potential model of Pt-Pd show that the tetrahedral structures can be stabilized for intermediate compositions of these nanoalloys, even when they are not the most stable structures of the elemental clusters. These results are also confirmed by density functional theory calculations for the magic sizes 59, 100, and 180. A thermodynamic analysis by the harmonic superposition approximation shows that Pt-Pd tetrahedral nanoalloys can be stable even above room temperature.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Ferrando R.Structure and Properties of Nanoalloys; Frontiers of Nanoscience; Vol. 10; Elsevier, 2016.
-
- Baletto F.; Ferrando R.; Fortunelli A.; Montalenti F.; Mottet C. Crossover among structural motifs in transition and noble-metal clusters. J. Chem. Phys. 2002, 116, 3856.10.1063/1.1448484. - DOI
-
- Marks L. D. Experimental studies of small-particle structures. Rep. Prog. Phys. 1994, 57, 603–649. 10.1088/0034-4885/57/6/002. - DOI
-
- Cleveland C. L.; Landman U.; Schaaff T. G.; Shafigullin M. N.; Stephens P. W.; Whetten R. L. Structural Evolution of Smaller Gold Nanocrystals: The Truncated Decahedral Motif. Phys. Rev. Lett. 1997, 79, 1873–1876. 10.1103/PhysRevLett.79.1873. - DOI
-
- Teranishi T.; Kurita R.; Miyake M. Shape Control of Pt Nanoparticles. Journal of Inorganic and Organometallic Polymers 2000, 10, 145–156. 10.1023/A:1009476128466. - DOI
LinkOut - more resources
Full Text Sources

