Engineering Sensory Ganglion Multicellular System to Model Tissue Nerve Ingrowth
- PMID: 38113315
- PMCID: PMC10953573
- DOI: 10.1002/advs.202308478
Engineering Sensory Ganglion Multicellular System to Model Tissue Nerve Ingrowth
Abstract
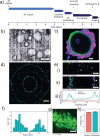
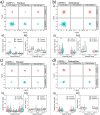
Discogenic pain is associated with deep nerve ingrowth in annulus fibrosus tissue (AF) of intervertebral disc (IVD). To model AF nerve ingrowth, primary bovine dorsal root ganglion (DRG) micro-scale tissue units are spatially organised around an AF explant by mild hydrodynamic forces within a collagen matrix. This results in a densely packed multicellular system mimicking the native DRG tissue morphology and a controlled AF-neuron distance. Such a multicellular organisation is essential to evolve populational-level cellular functions and in vivo-like morphologies. Pro-inflammatory cytokine-primed AF demonstrates its neurotrophic and neurotropic effects on nociceptor axons. Both effects are dependent on the AF-neuron distance underpinning the role of recapitulating inter-tissue/organ anatomical proximity when investigating their crosstalk. This is the first in vitro model studying AF nerve ingrowth by engineering mature and large animal tissues in a morphologically and physiologically relevant environment. The new approach can be used to biofabricate multi-tissue/organ models for untangling pathophysiological conditions and develop novel therapies.
Keywords: acoustic assembly; advanced in vitro models; alternatives to animal testing; multicellular system; sensory nerve ingrowth.
© 2023 AO RESEARCH INSTITUTE DAVOS (ARI). Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Madsen J. E., Hukkanen M., Aune A. K., Basran I., Moller J. F., Polak J. M., Nordsletten L., Clin. Orthop. Relat. Res. 1998, 351, 230. - PubMed
-
- Ackermann P. W., Franklin S. L., Dean B. J. F., Carr A. J., Salo P. T., Hart D. A., Front. Biosci. FRONT BIOSCI‐LANDMRK 2014, 19, 1251. - PubMed
-
- Mapp P. I., Walsh D. A., Nat. Rev. Rheumatol. 2012, 8, 390. - PubMed
-
- a) Peng B., Hao J., Hou S., Wu W., Jiang D., Fu X., Yang Y., Spine 2006, 31, 560; - PubMed
- b) Peng B., Wu W., Hou S., Li P., Zhang C., Yang Y., Journal of Bone & Joint Surgery, British Volume 2005, 87‐B (1), 62; - PubMed
- c) Johnson W. E., Evans H., Menage J., Eisenstein S. M., El Haj A., Roberts S., Spine 2001, 26, 2550. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
