Low RNA disruption during neoadjuvant chemotherapy predicts pathologic complete response absence in patients with breast cancer
- PMID: 38113421
- PMCID: PMC10765091
- DOI: 10.1093/jncics/pkad107
Low RNA disruption during neoadjuvant chemotherapy predicts pathologic complete response absence in patients with breast cancer
Abstract
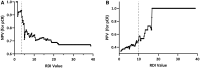
In previously reported retrospective studies, high tumor RNA disruption during neoadjuvant chemotherapy predicted for post-treatment pathologic complete response (pCR) and improved disease-free survival at definitive surgery for primary early breast cancer. The BREVITY (Breast Cancer Response Evaluation for Individualized Therapy) prospective clinical trial (NCT03524430) seeks to validate these prior findings. Here we report training set (Phase I) findings, including determination of RNA disruption index (RDI) cut points for outcome prediction in the subsequent validation set (Phase II; 454 patients). In 80 patients of the training set, maximum tumor RDI values for biopsies obtained during neoadjuvant chemotherapy were significantly higher in pCR responders than in patients without pCR post-treatment (P = .008). Moreover, maximum tumor RDI values ≤3.7 during treatment predicted for a lack of pCR at surgery (negative predictive value = 93.3%). These findings support the prospect that on-treatment tumor RNA disruption assessments may effectively predict post-surgery outcome, possibly permitting treatment optimization.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
The authors disclose the following conflicts of interest. A.P. and M.T. are minority shareholders in Rna Diagnostics, Inc. A.P. also serves as the company’s Chief Scientific Officer. L.P. is an employee of Rna Diagnostics, Inc, and is its Vice President of Research and Development. S.N. is also an employee of Rna Diagnostics and serves as its Vice President of Clinical Development. None of the remaining authors declare conflicts of interest related to the content of this article.
Figures
References
-
- Albain KS, Barlow WE, Ravdin PM, et al.; Breast Cancer Intergroup of North America. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9707):2055-2063. - PMC - PubMed
-
- Di Nardo P, Lisanti C, Garutti M, et al.Chemotherapy in patients with early breast cancer: clinical overview and management of long-term side effects. Expert Opin Drug Saf. 2022;21(11):1341-1355. - PubMed
-
- Baroudjian B, Arangalage D, Cuzzubbo S, et al.; PATIO group. Management of immune-related adverse events resulting from immune checkpoint blockade. Expert Rev Anticancer Ther. 2019;19(3):209-222. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
