Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax
- PMID: 38113472
- PMCID: PMC10877112
- DOI: 10.1182/bloodadvances.2023011757
Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax
Abstract
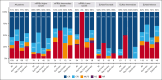
Hypomethylating agents (HMAs) and venetoclax (Ven) represent the standard of care for patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy. However, the European LeukemiaNet (ELN) risk classifications have been validated for patients treated with intensive therapy. In this study, we validate a recently proposed new molecular prognostic risk signature (mPRS) for patients with AML treated with HMAs and Ven. This classification allocated patients to favorable, intermediate (N/KRAS or FLT3-internal tandem duplication mutations), and lower (TP53 mutations) benefit groups. We retrospectively analyzed 159 patients treated with HMA and Ven. The mPRS classification allocated 74 (47%), 31 (19%), and 54 (34%) patients to the higher, intermediate, and lower-benefit groups, respectively. The overall response rate was 71% (86%, 54%, and 59% in the higher, intermediate, and lower-benefit groups, respectively). The median overall survival (OS) and event-free survival (EFS) times were 30 and 19 months, respectively, in the higher-benefit group; 12 and 8 months in the intermediate-benefit group; and 5 and 4 months in the lower-benefit group (P < .001). The C-index for OS and EFS was higher when stratifying patients according to mPRS classification than with the ELN 2022 classification. The 2-year cumulative incidence of relapse was 35%, 70%, and 60% in the higher, intermediate, and lower-benefit groups, respectively (P = .005). The mPRS classification accurately segregated groups of patients with AML treated with HMA plus Ven. In these patients, N/KRAS and TP53 mutations appear to negatively affect outcomes; therefore, new treatment approaches are warranted.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.D.D. has been a board of directors or advisory committee member for Genmab, GlaxoSmithKline, Kura Oncology, and Notable Labs; has received honoraria from Kura, Astellas Pharma, bluebird bio, Bristol Myers Squibb, Foghorn Therapeutics, Immune-Onc Therapeutics, Novartis, Takeda Oncology, Gilead Sciences, and Jazz Pharmaceuticals; is a current holder of stock options for Notable Labs; has been a consultant for AbbVie and Servier; and has received research funding from Servier, Bristol Myers Squibb, Foghorn, Immune-Onc Therapeutics, Loxo Oncology, Astex Pharmaceuticals, Cleave, and Forma. A.M. reports support from BioSight, Sanofi, and Astex Pharmaceuticals. G.B. has received research funding from Astex Pharmaceuticals, Ryvu Therapeutics, and PTC Therapeutics; has been a board of directors or advisory committee member for Pacyclex Pharmaceuticals, Novartis, CytomX, and Bio Ascend; and has been a consultant for Catamaran Bio, AbbVie, PPD Development, Protagonist Therapeutics, and Janssen. N.S. has been a consultant for Takeda Oncology, AstraZeneca, Amgen, Novartis, and Pfizer; received research funding from Takeda Oncology, Astellas, and Stemline Therapeutics; and received honoraria from Amgen. K.T. has been a consultant for SymBio Pharmaceuticals; and received honoraria from Mission Bio, Illumina, and Otsuka Pharmaceutical. N.G.D. has received research funding from Astellas Pharma, AbbVie, Genentech, Daiichi Sankyo, Gilead Sciences, ImmunoGen, Pfizer, BristolMyers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi Pharm, Fate Therapeutics, Amgen, Kite Pharma, Novartis, Astex Pharmaceuticals, KAHR, Shattuck, Sobi, GlycoMimetics, and Trillium; has been an adviser for Astellas Pharma, AbbVie, Genentech, Daiichi Sankyo, Novartis, Jazz Pharmaceuticals, Amgen, Servier, Karyopharm Therapeutics, Trovagene, Trillium, Syndax, Gilead Sciences, Pfizer, Bristol Myers Squibb, Kite Pharma, Actinium Pharmaceuticals, Arog Pharmaceuticals, ImmunoGen, Arcellx, and Shattuck; has been a data-monitoring committee member for Kartos Therapeutics and Jazz Pharmaceuticals; has been a consultant or board of directors or advisory committee member for Agios, Celgene, Sobi, and STAR Therapeutics; and has received research funding from Karyopham Therapeutics and Newave Pharmaceutical. G.I. has been a consultant for Novartis, Kura Oncology, and NuProbe and received research funding from Celgene, Kura Oncology, Syndax, Merck, Cullinan Oncology, and Novartis. N.P. has been a consultant for AbbVie, Astellas Pharma, Cimeio Therapeutics, CTI BioPharma, ImmunoGen, Intellisphere, Patient Power, PharmaEssentia, Protagonist Therapeutics, and TotalCME; has been a scientific/advisory committee member for Blueprint Medicines, Cancer.Net, CareDx, CTI BioPharma, Incyte, Menarini Group, and Pacylex Pharmaceuticals; has been a speaker or held a preceptorship for AbbVie, the Aplastic Anemia & MDS International Foundation, Curio Bioscience, DAVA Oncology, Intellisphere, Magdalen Medical Publishing, Medscape, Novartis, the Physician's Education Resource, Protagonist Therapeutics, and Targeted Oncology; and has received research support from the US Department of Defense. M.Y. has received research funding from Daiichi-Sankyo and Pfizer. G.G.-M. has received research funding from Astex Pharmaceuticals, Novartis, AbbVie, Bristol Myers Squibb, Genentech, Aprea Therapeutics, Curis, and Gilead Sciences; has been a consultant for Astex Pharmaceuticals, Acceleron Pharma, and Bristol Myers Squibb; and has received honoraria from Astex Pharmaceuticals, Acceleron Pharma, AbbVie, Novartis, Gilead Sciences, Curis, Genentech, and Bristol Myers Squibb. F.R. has received research funding from Amgen, Astex Pharmaceuticals/Taiho Oncology, Bristol Myers Squibb/Celgene, Syos, AbbVie, Prelude, Xencor, Astellas Pharma, and Biomea Fusion; honoraria from Amgen, Bristol Myers Squibb/Celgene, Syos, AbbVie, and Astellas Pharma; has been a board of directors or advisory committee member for Astex Pharmaceuticals/Taiho Oncology; and has been a consultant for Bristol Myers Squibb/Celgene, Syos, Novartis, AbbVie, AstraZeneca, and Astellas Pharma. H.M.K. has received research funding from AbbVie, Amgen, Ascentage Pharma, Bristol Myers Squibb, Daiichi Sankyo, ImmunoGen, Jazz Pharmaceuticals, and Novartis as well as honoraria from AbbVie, Amgen, Amphista Therapeutics, Ascentage Pharma, Astellas Pharma, Biologix, Curis, Ipsen, KAHR, Novartis, Pfizer, Precision BioSciences, Shenzhen TargetRx, and Takeda Oncology. T.M.K. has been a consultant for AbbVie, Agios, Bristol Myers Squibb, Genentech, Jazz Pharmaceuticals, Novartis, Servier, and PinotBio; has received research funding from AbbVie, Bristol Myers Squibb, Genentech, Jazz Pharmaceuticals, Pfizer, Cellenkos, Ascentage Pharma, Galectin Therapeutics, Astellas Pharma, AstraZeneca, Amgen, Cyclacel Pharmaceuticals, Delta-Fly Pharma, Iterion Therapeutics, GlycoMimetics, and Regeneron Pharmaceuticals; and has received honoraria from Astex Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures



References
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. - PubMed
-
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an International Expert Panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. - PubMed
-
- Arellano M, Carlisle JW. How I treat older patients with acute myeloid leukemia. Cancer. 2018;124(12):2472–2483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

