Dapagliflozin treatment is associated with a reduction of epicardial adipose tissue thickness and epicardial glucose uptake in human type 2 diabetes
- PMID: 38115004
- PMCID: PMC10731727
- DOI: 10.1186/s12933-023-02091-0
Dapagliflozin treatment is associated with a reduction of epicardial adipose tissue thickness and epicardial glucose uptake in human type 2 diabetes
Abstract
Objective: We recently demonstrated that treatment with sodium-glucose cotransporter-2 inhibitors (SGLT-2i) leads to an increase in myocardial flow reserve in patients with type 2 diabetes (T2D) with stable coronary artery disease (CAD). The mechanism by which this occurs is, however, unclear. One of the risk factors for cardiovascular disease is inflammation of epicardial adipose tissue (EAT). Since the latter is often increased in type 2 diabetes patients, it could play a role in coronary microvascular dysfunction. It is also well known that SGLT-2i modify adipose tissue metabolism. We aimed to investigate the effects of the SGLT-2i dapagliflozin on metabolism and visceral and subcutaneous adipose tissue thickness in T2D patients with stable coronary artery disease and to verify whether these changes could explain observed changes in myocardial flow.
Methods: We performed a single-center, prospective, randomized, double-blind, controlled clinical trial with 14 T2D patients randomized 1:1 to SGLT-2i dapagliflozin (10 mg daily) or placebo. The thickness of visceral (epicardial, mediastinal, perirenal) and subcutaneous adipose tissue and glucose uptake were assessed at baseline and 4 weeks after treatment initiation by 2-deoxy-2-[18F]fluoro-D-glucose Positron Emission Tomography/Computed Tomography during hyperinsulinemic euglycemic clamp.
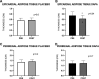
Results: The two groups were well-matched for baseline characteristics (age, diabetes duration, HbA1c, BMI, renal and heart function). Dapagliflozin treatment significantly reduced EAT thickness by 19% (p = 0.03). There was a significant 21.6% reduction in EAT glucose uptake during euglycemic hyperinsulinemic clamp in the dapagliflozin group compared with the placebo group (p = 0.014). There were no significant effects on adipose tissue thickness/metabolism in the other depots explored.
Conclusions: SGLT-2 inhibition selectively reduces EAT thickness and EAT glucose uptake in T2D patients, suggesting a reduction of EAT inflammation. This could explain the observed increase in myocardial flow reserve, providing new insights into SGLT-2i cardiovascular benefits.
Trial registration: ClinicalTrials.gov NCT03313752.
Keywords: Diabetes; Epicardial adipose tissue; Metabolism; Microvascular dysfunction; PET; Precision medicine; SGLT-2i.
© 2023. The Author(s).
Conflict of interest statement
None of the authors have any competing interests in the manuscript.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

