Preclinical assessment of an anti-HTLV-1 heterologous DNA/MVA vaccine protocol expressing a multiepitope HBZ protein
- PMID: 38115107
- PMCID: PMC10731796
- DOI: 10.1186/s12985-023-02264-z
Preclinical assessment of an anti-HTLV-1 heterologous DNA/MVA vaccine protocol expressing a multiepitope HBZ protein
Abstract
Background: Human T-lymphotropic virus 1 (HTLV-1) is associated with the development of several pathologies and chronic infection in humans. The inefficiency of the available treatments and the challenge in developing a protective vaccine highlight the need to produce effective immunotherapeutic tools. The HTLV-1 basic leucine zipper (bZIP) factor (HBZ) plays an important role in the HTLV-1 persistence, conferring a survival advantage to infected cells by reducing the HTLV-1 proteins expression, allowing infected cells to evade immune surveillance, and enhancing cell proliferation leading to increased proviral load.
Methods: We have generated a recombinant Modified Virus Vaccinia Ankara (MVA-HBZ) and a plasmid DNA (pcDNA3.1(+)-HBZ) expressing a multiepitope protein based on peptides of HBZ to study the immunogenic potential of this viral-derived protein in BALB/c mice model. Mice were immunized in a prime-boost heterologous protocol and their splenocytes (T CD4+ and T CD8+) were immunophenotyped by flow cytometry and the humoral response was evaluated by ELISA using HBZ protein produced in prokaryotic vector as antigen.
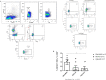
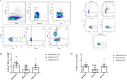
Results: T CD4+ and T CD8+ lymphocytes cells stimulated by HBZ-peptides (HBZ42-50 and HBZ157-176) showed polyfunctional double positive responses for TNF-α/IFN-γ, and TNF-α/IL-2. Moreover, T CD8+ cells presented a tendency in the activation of effector memory cells producing granzyme B (CD44+High/CD62L-Low), and the activation of Cytotoxic T Lymphocytes (CTLs) and cytotoxic responses in immunized mice were inferred through the production of granzyme B by effector memory T cells and the expression of CD107a by CD8+ T cells. The overall data is consistent with a directive and effector recall response, which may be able to operate actively in the elimination of HTLV-1-infected cells and, consequently, in the reduction of the proviral load. Sera from immunized mice, differently from those of control animals, showed IgG-anti-HBZ production by ELISA.
Conclusions: Our results highlight the potential of the HBZ multiepitope protein expressed from plasmid DNA and a poxviral vector as candidates for therapeutic vaccine.
Keywords: HBZ; Human T-lymphotropic virus 1; Plasmid DNA vaccine (pcDNA3.1(+)-HBZ); Recombinant Modified Vaccinia virus Ankara (MVA-HBZ); Vaccine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Proietti ABFC. Cadernos hemominas—HTLV. 6. Belo Horizonte: Fundação Hemominas; 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

