Senescent skeletal muscle fibroadipogenic progenitors recruit and promote M2 polarization of macrophages
- PMID: 38115574
- PMCID: PMC10928562
- DOI: 10.1111/acel.14069
Senescent skeletal muscle fibroadipogenic progenitors recruit and promote M2 polarization of macrophages
Abstract
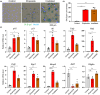
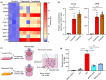
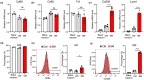
Senescent cells compromise tissue structure and function in older organisms. We recently identified senescent fibroadipogenic progenitors (FAPs) with activated chemokine signaling pathways in the skeletal muscle of old mice, and hypothesized these cells may contribute to the age-associated accumulation of immune cells in skeletal muscle. In this study, through cell-cell communication analysis of skeletal muscle single-cell RNA-sequencing data, we identified unique interactions between senescent FAPs and macrophages, including those mediated by Ccl2 and Spp1. Using mouse primary FAPs in vitro, we verified increased expression of Ccl2 and Spp1 and secretion of their respective proteins in the context of both irradiation- and etoposide-induced senescence. Compared to non-senescent FAPs, the medium of senescent FAPs markedly increased the recruitment of macrophages in an in vitro migration assay, an effect that was mitigated by preincubation with antibodies to either CCL2 or osteopontin (encoded by Spp1). Further studies demonstrated that the secretome of senescent FAPs promotes polarization of macrophages toward an M2 subtype. These data suggest the unique secretome of senescent FAPs may compromise skeletal muscle homeostasis by recruiting and directing the behavior of macrophages.
Keywords: cellular senescence; fibroadipogenic progenitors (FAPs); macrophages; migration; polarization.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Chambers, E. S. , Vukmanovic‐Stejic, M. , Shih, B. B. , Trahair, H. , Subramanian, P. , Devine, O. P. , Glanville, J. , Gilroy, D. , Rustin, M. H. A. , Freeman, T. C. , Mabbott, N. A. , & Akbar, A. N. (2021). Recruitment of inflammatory monocytes by senescent fibroblasts inhibits antigen‐specific tissue immunity during human aging. Nature Aging, 1, 101–113. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

