Mulibrey nanism and immunological complications: a comprehensive case report and literature review
- PMID: 38116000
- PMCID: PMC10728670
- DOI: 10.3389/fimmu.2023.1303251
Mulibrey nanism and immunological complications: a comprehensive case report and literature review
Abstract
Introduction: Mulibrey nanism (MUL) is a rare disorder caused by TRIM37 gene variants characterized by growth failure, dysmorphic features, congestive heart failure (CHF), and an increased risk of Wilms' tumor. Although immune system impairment has been documented in MUL, the underlying mechanisms remain poorly understood.
Methods: We present a case of MUL with progressive lymphopenia and review similar cases from the literature.
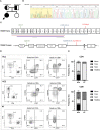
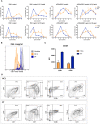
Results: Our patient presented with prenatal onset growth restriction, characteristic dysmorphic features, and Wilms' tumor. She developed progressive lymphopenia starting at 10 years of age, leading to the initiation of intravenous immunoglobulin (IVIG) replacement therapy and infection prophylaxis. Genetic analysis detected a likely pathogenic variant on the maternal allele and copy number loss on the paternal allele in TRIM37. Subsequently a cardiac magnetic resonance imaging was conducted revealing signs of pericardial constriction raising concerns for intestinal lymphatic losses. The cessation of IVIG therapy did not coincide with any increase in the rate of infections. The patient exhibited a distinct immunological profile, characterized by hypogammaglobulinemia, impaired antibody responses, and skewed T-cell subsets with an altered CD4+/CD8+ ratio, consistent with previous reports. Normal thymocyte development assessed by artificial thymic organoid platform ruled out an early hematopoietic intrinsic defect of T-cell development.
Discussion: The immunological profile of MUL patients reported so far shares similarities with that described in protein-losing enteropathy secondary to CHF in Fontan circulation and primary intestinal lymphangiectasia. These similarities include hypogammaglobulinemia, significant T-cell deficiency with decreased CD4+ and CD8+ counts, altered CD4+/CD8+ ratios, and significantly modified CD4+ and CD8+ T-cell phenotypes toward effector and terminal differentiated T cells, accompanied by a loss of naïve CD45RA+ T lymphocytes. In MUL, CHF is a cardinal feature, occurring in a significant proportion of patients and influencing prognosis. Signs of CHF or constrictive pericarditis have been evident in the case reported here and in all cases of MUL with documented immune dysfunction reported so far. These observations raise intriguing connections between these conditions. However, further investigation is warranted to in-depth define the immunological defect, providing valuable insights into the pathophysiology and treatment strategies for this condition.
Keywords: CD4+ lymphopenia; Mulibrey; case report; hypogammaglobulinemia; pericardial constriction.
Copyright © 2023 Gazzin, Pala, Bosticardo, Niemela, Stoddard, Biasin, Quarello, Carli, Ferroni, Delmonte, Montin, Rosenzweig, Licciardi and Notarangelo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

