Engineering Novosphingobium aromaticivorans to produce cis,cis-muconic acid from biomass aromatics
- PMID: 38117061
- PMCID: PMC10807440
- DOI: 10.1128/aem.01660-23
Engineering Novosphingobium aromaticivorans to produce cis,cis-muconic acid from biomass aromatics
Abstract
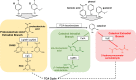
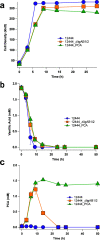
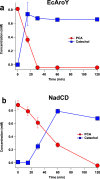
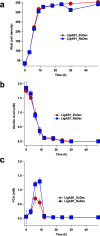
The platform chemical cis,cis-muconic acid (ccMA) provides facile access to a number of monomers used in the synthesis of commercial plastics. It is also a metabolic intermediate in the β-ketoadipic acid pathway of many bacteria and, therefore, a current target for microbial production from abundant renewable resources via metabolic engineering. This study investigates Novosphingobium aromaticivorans DSM12444 as a chassis for the production of ccMA from biomass aromatics. The N. aromaticivorans genome predicts that it encodes a previously uncharacterized protocatechuic acid (PCA) decarboxylase and a catechol 1,2-dioxygenase, which would be necessary for the conversion of aromatic metabolic intermediates to ccMA. This study confirmed the activity of these two enzymes in vitro and compared their activity to ones that have been previously characterized and used in ccMA production. From these results, we generated one strain that is completely derived from native genes and a second that contains genes previously used in microbial engineering synthesis of this compound. Both of these strains exhibited stoichiometric production of ccMA from PCA and produced greater than 100% yield of ccMA from the aromatic monomers that were identified in liquor derived from alkaline pretreated biomass. Our results show that a strain completely derived from native genes and one containing homologs from other hosts are both capable of stoichiometric production of ccMA from biomass aromatics. Overall, this work combines previously unknown aspects of aromatic metabolism in N. aromaticivorans and the genetic tractability of this organism to generate strains that produce ccMA from deconstructed biomass.IMPORTANCEThe production of commodity chemicals from renewable resources is an important goal toward increasing the environmental and economic sustainability of industrial processes. The aromatics in plant biomass are an underutilized and abundant renewable resource for the production of valuable chemicals. However, due to the chemical composition of plant biomass, many deconstruction methods generate a heterogeneous mixture of aromatics, thus making it difficult to extract valuable chemicals using current methods. Therefore, recent efforts have focused on harnessing the pathways of microorganisms to convert a diverse set of aromatics into a single product. Novosphingobium aromaticivorans DSM12444 has the native ability to metabolize a wide range of aromatics and, thus, is a potential chassis for conversion of these abundant compounds to commodity chemicals. This study reports on new features of N. aromaticivorans that can be used to produce the commodity chemical cis,cis-muconic acid from renewable and abundant biomass aromatics.
Keywords: Novosphingobium; aromatic compounds; carbon metabolism; decarboxylases; extradiol; intradiol; lignin; metabolic engineering; muconic acid; sphingomonads.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

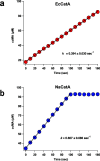


References
-
- Shanks BH, Keeling PL. 2017. Bioprivileged molecules: creating value from biomass. Green Chem 19:3177–3185. doi:10.1039/C7GC00296C - DOI
-
- Sonoki T, Takahashi K, Sugita H, Hatamura M, Azuma Y, Sato T, Suzuki S, Kamimura N, Masai E. 2018. Glucose-free cis,cis-muconic acid production via new metabolic designs corresponding to the heterogeneity of lignin. ACS Sustainable Chem Eng 6:1256–1264. doi:10.1021/acssuschemeng.7b03597 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

