Mechanical Disruption of the Inner Limiting Membrane In Vivo Enhances Targeting to the Inner Retina
- PMID: 38117244
- PMCID: PMC10741092
- DOI: 10.1167/iovs.64.15.25
Mechanical Disruption of the Inner Limiting Membrane In Vivo Enhances Targeting to the Inner Retina
Abstract
Purpose: To evaluate the effects of mechanical disruption of the inner limiting membrane (ILM) on the ability to target interventions to the inner neurosensory retina in a rodent model. Our study used an animal model to gain insight into the normal physiology of the ILM and advances our understanding of the effects of mechanical ILM removal on the viral transduction of retinal ganglion cells and retinal ganglion cell transplantation.
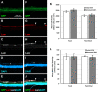
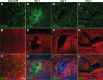
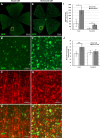
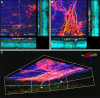
Methods: The ILM in the in vivo rat eye was disrupted using mechanical forces applied to the vitreoretinal interface. Immunohistology and electron microscopy were used to verify the removal of the ILM in retina flatmounts and sections. To assess the degree to which ILM disruption enhanced transvitreal access to the retina, in vivo studies involving intravitreal injections of adeno-associated virus (AAV) to transduce retinal ganglion cells (RGCs) and ex vivo studies involving co-culture of human stem cell-derived RGCs (hRGCs) on retinal explants were performed. RGC transduction efficiency and transplanted hRGC integration with retinal explants were evaluated by immunohistology of the retinas.
Results: Mechanical disruption of the ILM in the rodent eye was sufficient to remove the ILM from targeted retinal areas while preserving the underlying retinal nerve fiber layer and RGCs. Removal of the ILM enhanced the transduction efficiency of intravitreally delivered AAV threefold (1380.0 ± 290.1 vs. 442.0 ± 249.3 cells/mm2; N = 6; P = 0.034). Removal of the ILM was also sufficient to promote integration of transplanted RGCs within the inner retina.
Conclusions: The ILM is a barrier to transvitreally delivered agents including viral vectors and cells. Mechanical removal of the ILM is sufficient to enhance access to the inner retina, improve viral transduction efficiencies of RGCs, and enhance cellular integration of transplanted RGCs with the retina.
Conflict of interest statement
Disclosure:
Figures