The Regulation of Fatty Acid Synthase by Exosomal miR-143-5p and miR-342-5p in Idiopathic Pulmonary Fibrosis
- PMID: 38117249
- PMCID: PMC11478129
- DOI: 10.1165/rcmb.2023-0232OC
The Regulation of Fatty Acid Synthase by Exosomal miR-143-5p and miR-342-5p in Idiopathic Pulmonary Fibrosis
Abstract
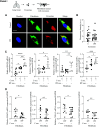
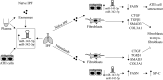
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive disease caused by an aberrant repair of injured alveolar epithelial cells. The maintenance of the alveolar epithelium and its regeneration after the damage is fueled by alveolar type II (ATII) cells. Injured cells release exosomes containing microRNAs (miRNAs), which can alter the recipient cells' function. Lung tissue, ATII cells, fibroblasts, plasma, and exosomes were obtained from naive patients with IPF, patients with IPF taking pirfenidone or nintedanib, and control organ donors. miRNA expression was analyzed to study their impact on exosome-mediated effects in IPF. High miR-143-5p and miR-342-5p levels were detected in ATII cells, lung tissue, plasma, and exosomes in naive patients with IPF. Decreased FASN (fatty acid synthase) and ACSL-4 (acyl-CoA-synthetase long-chain family member 4) expression was found in ATII cells. miR-143-5p and miR-342-5p overexpression or ATII cell treatment with IPF-derived exosomes containing these miRNAs lowered FASN and ACSL-4 levels. Also, this contributed to ATII cell injury and senescence. However, exosomes isolated from patients with IPF taking nintedanib or pirfenidone increased FASN expression in ATII cells compared with naive patients with IPF. Furthermore, fibroblast treatment with exosomes obtained from naive patients with IPF increased SMAD3, CTGF, COL3A1, and TGFβ1 expression. Our results suggest that IPF-derived exosomes containing miR-143-5p and miR-342-5p inhibited the de novo fatty acid synthesis pathway in ATII cells. They also induced the profibrotic response in fibroblasts. Pirfenidone and nintedanib improved ATII cell function and inhibited fibrogenesis. This study highlights the importance of exosomes in IPF pathophysiology.
Keywords: IPF; alveolar type II cells; exosomes; fatty acid synthesis; microRNA.
Figures


















Comment in
-
Alveolar Epithelial Cell Dysfunction in Idiopathic Pulmonary Fibrosis Linked to Lipid Alterations: Therapeutic Implications.Am J Respir Cell Mol Biol. 2024 Apr;70(4):233-234. doi: 10.1165/rcmb.2023-0432ED. Am J Respir Cell Mol Biol. 2024. PMID: 38271680 Free PMC article. No abstract available.
References
-
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. - PubMed
-
- Sharif R. Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am J Manag Care . 2017;23:S176–S182. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

