Refocusing generalized myasthenia gravis: Patient burden, disease profiles, and the role of evolving therapy
- PMID: 38117543
- PMCID: PMC11236062
- DOI: 10.1111/ene.16180
Refocusing generalized myasthenia gravis: Patient burden, disease profiles, and the role of evolving therapy
Abstract
Background and purpose: Generalized myasthenia gravis (gMG) continues to present significant challenges for clinical management due to an unpredictable disease course, frequent disease fluctuations, and varying response to therapy. The recent availability of new pharmacologic therapies presents a valuable opportunity to reevaluate how this disease is classified, assessed, and managed and identify new ways to improve the clinical care of patients with gMG.
Methods: Narrative review was made of publications identified via searches of PubMed and selected congresses (January 2000-September 2022).
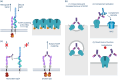
Results: New consensus definitions are required to ensure consistency, to better characterize patients, and to identify patients who will benefit from specific drugs and earlier use of these agents. There is a need for more frequent, standardized patient assessment to identify the cause of motor function deficits, provide a clearer picture of the disease burden and its impact on daily living and quality of life (QoL), and better support treatment decision-making. Novel approaches that target different components of the immune system will play a role in more precise treatment of patients with gMG, alongside the development of new algorithms to guide individualized patient management.
Conclusions: gMG has a physical, mental, and social impact, resulting in a considerable burden of disease and substantially decreased QoL, despite standard treatments. The availability of novel, targeted treatments that influence key pathological mediators of gMG, together with new biomarkers, offers the potential to optimize patient management and ultimately enables a greater number of patients to achieve minimal manifestation status and a reduced burden of disease.
Keywords: classification; disease burden; generalized myasthenia gravis; pathophysiology; targeted therapy.
© 2023 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
E.C.‐V. has received public speaking honoraria and compensation for advisory boards and/or consultation fees from argenx and UCB. S.J. has served as an international advisory board member for Alexion, Alnylam, argenx, Immunovant, Regeneron, and UCB; is currently an expert panel member of the Myasthenia Gravis Consortium for argenx; and has received speaker fees from Eisai Pharmaceuticals and Terumo BCT. F.S. has received public speaking honoraria from Alexion Pharmaceuticals, Biogen, Mylan, Novartis, Roche Pharma, Sanofi, and Teva Pharmaceuticals; he has also received compensation for advisory boards and/or consultation fees from Alexion Pharmaceuticals, Almirall, argenx, AveXis, Biogen, Forward Pharma, Lexeo Therapeutics, Merck, Novartis, Novatek, Pomona, Roche, Sanofi, and Takeda and is currently the principal investigator in clinical trials sponsored by Alexion Pharmaceuticals, argenx, Prilenia, and Sanofi. E.S.‐C. has received public speaking honoraria from Biogen and Sanofi; she has also received compensation for advisory boards and/or consultation fees from Amicus, argenx, Biogen, Lupin, Roche, and Sanofi. CS.‐G. has received public speaking honoraria and/or compensation for advisory boards/consultation fees from Alexion Pharmaceuticals, Amicus Therapeutics, argenx, Bayer Schering, Hormosan Pharma, Immunovant, Janssen, Lupin Pharmaceuticals, Roche Pharma, Teva Pharmaceuticals, and UCB.
Figures
References
-
- Jacob S. Refractory myasthenia gravis: patient burden and the need for new therapeutic targets. Eur Neurol Rev. 2018;13(1):18‐20.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

