Transferrin receptor in primary and metastatic breast cancer: Evaluation of expression and experimental modulation to improve molecular targeting
- PMID: 38117806
- PMCID: PMC10732420
- DOI: 10.1371/journal.pone.0293700
Transferrin receptor in primary and metastatic breast cancer: Evaluation of expression and experimental modulation to improve molecular targeting
Abstract
Background: Conjugation of transferrin (Tf) to imaging or nanotherapeutic agents is a promising strategy to target breast cancer. Since the efficacy of these biomaterials often depends on the overexpression of the targeted receptor, we set out to survey expression of transferrin receptor (TfR) in primary and metastatic breast cancer samples, including metastases and relapse, and investigate its modulation in experimental models.
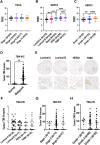
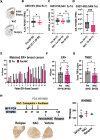
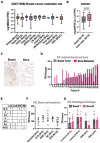
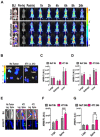
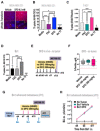
Methods: Gene expression was investigated by datamining in twelve publicly-available datasets. Dedicated Tissue microarrays (TMAs) were generated to evaluate matched primary and bone metastases as well as and pre and post chemotherapy tumors from the same patient. TMA were stained with the FDA-approved MRQ-48 antibody against TfR and graded by staining intensity (H-score). Patient-derived xenografts (PDX) and isogenic metastatic mouse models were used to study in vivo TfR expression and uptake of transferrin.
Results: TFRC gene and protein expression were high in breast cancer of all subtypes and stages, and in 60-85% of bone metastases. TfR was detectable after neoadjuvant chemotherapy, albeit with some variability. Fluorophore-conjugated transferrin iron chelator deferoxamine (DFO) enhanced TfR uptake in human breast cancer cells in vitro and proved transferrin localization at metastatic sites and correlation of tumor burden relative to untreated tumor mice.
Conclusions: TfR is expressed in breast cancer, primary, metastatic, and after neoadjuvant chemotherapy. Variability in expression of TfR suggests that evaluation of the expression of TfR in individual patients could identify the best candidates for targeting. Further, systemic iron chelation with DFO may upregulate receptor expression and improve uptake of therapeutics or tracers that use transferrin as a homing ligand.
Copyright: © 2023 Fontana et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
no authors have competing interests
Figures


References
-
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al.. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018; 391: 1023–75. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

