Highly specific σ2R/TMEM97 ligand FEM-1689 alleviates neuropathic pain and inhibits the integrated stress response
- PMID: 38117854
- PMCID: PMC10756276
- DOI: 10.1073/pnas.2306090120
Highly specific σ2R/TMEM97 ligand FEM-1689 alleviates neuropathic pain and inhibits the integrated stress response
Abstract
The sigma 2 receptor (σ2R) was described pharmacologically more than three decades ago, but its molecular identity remained obscure until recently when it was identified as transmembrane protein 97 (TMEM97). We and others have shown that σ2R/TMEM97 ligands alleviate mechanical hypersensitivity in mouse neuropathic pain models with a time course wherein maximal antinociceptive effect is approximately 24 h following dosing. We sought to understand this unique antineuropathic pain effect by addressing two key questions: do these σ2R/TMEM97 compounds act selectively via the receptor, and what is their downstream mechanism on nociceptive neurons? Using male and female conventional knockout mice for Tmem97, we find that a σ2R/TMEM97 binding compound, FEM-1689, requires the presence of the gene to produce antinociception in the spared nerve injury model in mice. Using primary mouse dorsal root ganglion neurons, we demonstrate that FEM-1689 inhibits the integrated stress response (ISR) and promotes neurite outgrowth via a σ2R/TMEM97-specific action. We extend the clinical translational value of these findings by showing that FEM-1689 reduces ISR and p-eIF2α levels in human sensory neurons and that it alleviates the pathogenic engagement of ISR by methylglyoxal. We also demonstrate that σ2R/TMEM97 is expressed in human nociceptors and satellite glial cells. These results validate σ2R/TMEM97 as a promising target for further development for the treatment of neuropathic pain.
Keywords: ISR; TMEM97; drug discovery; pain; sigma 2 receptor.
Conflict of interest statement
Competing interests statement:M.S.Y., J.J.S., S.F.M., and T.J.P. are co-founders of NuvoNuro, Inc. S.F.M. and J.J.S. report being co-inventors on patents and pending patent applications related to work described in this article.
Figures
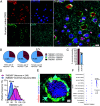
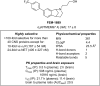


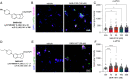
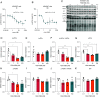


Update of
-
Highly specific σ2R/TMEM97 ligand alleviates neuropathic pain and inhibits the integrated stress response.bioRxiv [Preprint]. 2023 Oct 17:2023.04.11.536439. doi: 10.1101/2023.04.11.536439. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2306090120. doi: 10.1073/pnas.2306090120. PMID: 37090527 Free PMC article. Updated. Preprint.
References
-
- Cassano G., et al. , F281, synthetic agonist of the sigma-2 receptor, induces Ca2+ efflux from the endoplasmic reticulum and mitochondria in SK-N-SH cells. Cell Calcium 45, 340–345 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

