Ir/Zn-cocatalyzed chemo- and atroposelective [2+2+2] cycloaddition for construction of C─N axially chiral indoles and pyrroles
- PMID: 38117883
- PMCID: PMC10732529
- DOI: 10.1126/sciadv.adk1704
Ir/Zn-cocatalyzed chemo- and atroposelective [2+2+2] cycloaddition for construction of C─N axially chiral indoles and pyrroles
Abstract
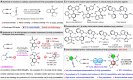
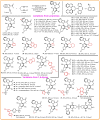
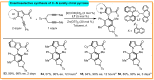
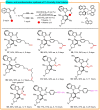
Here, an Ir/Zn-cocatalyzed atroposelective [2+2+2] cycloaddition of 1,6-diynes and ynamines was developed, forging various functionalized C─N axially chiral indoles and pyrroles in generally good to excellent yields (up to 99%), excellent chemoselectivities, and high enantioselectivities (up to 98% enantiomeric excess) with wide substrate scope. This cocatalyzed strategy not only provided an alternative promising and reliable way for asymmetric alkyne [2+2+2] cyclotrimerization in an easy handle but also settled the issues of previous [Rh(COD)2]BF4-catalyzed system on the construction of C─N axial chirality such as complex operations, limited substrate scope, and low efficiency. In addition, control experiments and theoretical calculations disclosed that Zn(OTf)2 markedly reduced the barrier of migration insertion to significantly increase reaction efficiency, which was distinctly different from previous work on the Lewis acid for improving reaction yield through accelerating oxidative addition and reductive elimination.
Figures

Similar articles
-
Enantioselective Rh-Catalyzed Azide-Internal-Alkyne Cycloaddition for the Construction of Axially Chiral 1,2,3-Triazoles.J Am Chem Soc. 2022 Apr 20;144(15):6981-6991. doi: 10.1021/jacs.2c01985. Epub 2022 Apr 8. J Am Chem Soc. 2022. PMID: 35394289
-
Organocatalytic diastereo- and atroposelective construction of N-N axially chiral pyrroles and indoles.Nat Commun. 2024 Jan 15;15(1):518. doi: 10.1038/s41467-024-44743-z. Nat Commun. 2024. PMID: 38225235 Free PMC article.
-
Organocatalytic Atroposelective Synthesis of N-N Axially Chiral Indoles and Pyrroles by De Novo Ring Formation.Angew Chem Int Ed Engl. 2022 Apr 19;61(17):e202116829. doi: 10.1002/anie.202116829. Epub 2022 Feb 23. Angew Chem Int Ed Engl. 2022. PMID: 35080808
-
Transition-metal-catalyzed atroposelective synthesis of axially chiral styrenes.Chem Commun (Camb). 2023 Oct 24;59(85):12669-12684. doi: 10.1039/d3cc03592a. Chem Commun (Camb). 2023. PMID: 37807950 Review.
-
A Strategy for Synthesizing Axially Chiral Naphthyl-Indoles: Catalytic Asymmetric Addition Reactions of Racemic Substrates.Angew Chem Int Ed Engl. 2019 Oct 14;58(42):15104-15110. doi: 10.1002/anie.201908279. Epub 2019 Sep 10. Angew Chem Int Ed Engl. 2019. PMID: 31441203 Review.
Cited by
-
Catalytic asymmetric intermolecular [4 + 2] annulation of benzocyclobutenones with Alkynes and activated carbonyls via C-C activation.Nat Commun. 2025 Jul 1;16(1):5741. doi: 10.1038/s41467-025-60109-5. Nat Commun. 2025. PMID: 40595458 Free PMC article.
-
Palladium-catalyzed redox diversified entry to axially chiral styrenes via asymmetric olefination with alkynes.Chem Sci. 2025 Jul 4;16(31):14252-14261. doi: 10.1039/d5sc04080a. eCollection 2025 Aug 6. Chem Sci. 2025. PMID: 40656525 Free PMC article.
-
Atroposelective Formal [2 + 5] Macrocyclization Synthesis for a Novel All-Hydrocarbon Cyclo[7] Meta-Benzene Macrocycle.Molecules. 2024 Jul 17;29(14):3363. doi: 10.3390/molecules29143363. Molecules. 2024. PMID: 39064941 Free PMC article.
-
Pathogenic Proteins Through the Lens of NMR Spectroscopy: Structural and Functional Insights into Disease.Cell Biochem Biophys. 2025 Aug 13. doi: 10.1007/s12013-025-01869-1. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40801977 Review. No abstract available.
References
-
- K. Cen, M. Usman, W. Shen, M. Liu, R. Yanga, J. Cai, A review on the assembly of multi-substituted pyridines via Co-catalyzed [2+2+2] cycloaddition with nitriles. Org. Biomol. Chem. 20, 7391–7404 (2022). - PubMed
-
- P. Matton, S. Huvelle, M. Haddad, P. Phansavath, V. Ratovelomanana-Vidal, Recent progress in metal-catalyzed [2+2+2] cycloaddition reactions. Synthesis 54, 4–32 (2022).
-
- T. Gläsel, B. N. Baumann, M. Hapke, Cobalt catalysts for [2+2+2] cycloaddition reactions: Isolated precatalysts and in situ generated catalysts. Chem. Rec. 21, 3727–3745 (2021). - PubMed
-
- M. Amatore, C. Aubert, Recent advances in stereoselective [2+2+2] cycloadditions. Eur. J. Org. Chem. 2015, 265–286 (2015).
-
- G. Nishida, K. Noguchi, M. Hirano, K. Tanaka, Enantioselective synthesis of P-stereogenic alkynylphosphine oxides by Rh-catalyzed [2+2+2] cycloaddition. Angew. Chem. Int. Ed. 47, 3410–3413 (2008). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

