A phage-encoded RNA-binding protein inhibits the antiviral activity of a toxin-antitoxin system
- PMID: 38117986
- PMCID: PMC10853763
- DOI: 10.1093/nar/gkad1207
A phage-encoded RNA-binding protein inhibits the antiviral activity of a toxin-antitoxin system
Abstract
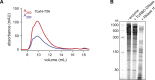
Bacteria harbor diverse mechanisms to defend themselves against their viral predators, bacteriophages. In response, phages can evolve counter-defense systems, most of which are poorly understood. In T4-like phages, the gene tifA prevents bacterial defense by the type III toxin-antitoxin (TA) system toxIN, but the mechanism by which TifA inhibits ToxIN remains unclear. Here, we show that TifA directly binds both the endoribonuclease ToxN and RNA, leading to the formation of a high molecular weight ribonucleoprotein complex in which ToxN is inhibited. The RNA binding activity of TifA is necessary for its interaction with and inhibition of ToxN. Thus, we propose that TifA inhibits ToxN during phage infection by trapping ToxN on cellular RNA, particularly the abundant 16S rRNA, thereby preventing cleavage of phage transcripts. Taken together, our results reveal a novel mechanism underlying inhibition of a phage-defensive RNase toxin by a small, phage-encoded protein.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Labrie S.J., Samson J.E., Moineau S.. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010; 8:317–327. - PubMed
-
- Tal N., Sorek R.. SnapShot: bacterial immunity. Cell. 2022; 185:578–578. - PubMed
-
- Bernheim A., Sorek R.. The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 2020; 18:113–119. - PubMed
-
- Hampton H.G., Watson B.N.J., Fineran P.C.. The arms race between bacteria and their phage foes. Nature. 2020; 577:327–336. - PubMed
-
- Samson J.E., Magadán A.H., Sabri M., Moineau S.. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 2013; 11:675–687. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

