Methodological Approach to Identify and Expand the Volume of Antimicrobial Resistance (AMR) Data in the Human Health Sector in Low- and Middle-Income Countries in Asia: Implications for Local and Regional AMR Surveillance Systems Strengthening
- PMID: 38118007
- PMCID: PMC10732564
- DOI: 10.1093/cid/ciad634
Methodological Approach to Identify and Expand the Volume of Antimicrobial Resistance (AMR) Data in the Human Health Sector in Low- and Middle-Income Countries in Asia: Implications for Local and Regional AMR Surveillance Systems Strengthening
Abstract
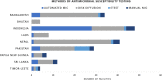
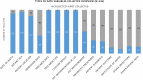
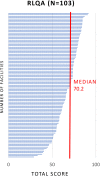
Antimicrobial resistance (AMR) is a multifaceted global health problem disproportionately affecting low- and middle-income countries (LMICs). The Capturing data on Antimicrobial resistance Patterns and Trends in Use in Regions of Asia (CAPTURA) project was tasked to expand the volume of AMR and antimicrobial use data in Asia. The CAPTURA project used 2 data-collection streams: facility data and project metadata. Project metadata constituted information collected to map out data sources and assess data quality, while facility data referred to the retrospective data collected from healthcare facilities. A down-selection process, labelled "the funnel approach" by the project, was adopted to use the project metadata in prioritizing and selecting laboratories for retrospective AMR data collection. Moreover, the metadata served as a guide for understanding the AMR data once they were collected. The findings from CAPTURA's metadata add to the current discourse on the limitation of AMR data in LMICs. There is generally a low volume of AMR data generated as there is a lack of microbiology laboratories with sufficient antimicrobial susceptibility testing capacity. Many laboratories in Asia are still capturing data on paper, resulting in scattered or unused data not readily accessible or shareable for analyses. There is also a lack of clinical and epidemiological data captured, impeding interpretation and in-depth understanding of the AMR data. CAPTURA's experience in Asia suggests that there is a wide spectrum of capacity and capability of microbiology laboratories within a country and region. As local AMR surveillance is a crucial instrument to inform context-specific measures to combat AMR, it is important to understand and assess current capacity-building needs while implementing activities to enhance surveillance systems.
Keywords: AMR surveillance; Antimicrobial Resistance (AMR); CAPTURA; capacity building; project implementation.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. Clark reports receiving funds for their institution from the CAPTURA grant. A. T. A. reports being an employee of Brigham and Women's Hospital since September 2022 and a consultant since 2021 and working as a consultant for the CAPTURA project from September 2021 to March 2022. A. S. reports receiving compensation from the Public Health Surveillance Group. W. M. reports receiving financial support from the International Vaccine Group, UKAID, and a subgrant for CAPTURA work. B. D. reports receiving compensation from the Public Health Surveillance Group. D. M. A. reports receiving financial support from the National Institute for Health and Care Research (NIHR) Global Health Research Unit on genomic surveillance of AMR and the Centre for Genomic Pathogen Surveillance. J. S. reports a subcontract for salary support and travel from the International Vaccine Institute. E. E. reports being a consultant with the International Vaccine Institute since April 2021 and staff and consulting funds from the WHO WPRO, ABD, FHI, and UNICEF. P. G. reports receiving compensation from the Public Health Surveillance Group. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures





References
-
- ONeill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. Wellcome Trust and HM Government. 2016.
-
- World Health Organization . Global action plan on antimicrobial resistance. Geneva, Switzerland: World Health Organization, 2015. - PubMed
-
- Fleming Fund. The Fleming Fund. Available at: https://www.flemingfund.org/ . Accessed 1 June 2023.
-
- Holm M, MacWright WR, Poudyal N, et al. Capturing data on Antimicrobial resistance Patterns and Trends in Use in Regions of Asia (CAPTURA). Clin Infect Dis 2023; 77(Suppl 7):S500–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

