Complex II ambiguities-FADH2 in the electron transfer system
- PMID: 38118236
- PMCID: PMC10772739
- DOI: 10.1016/j.jbc.2023.105470
Complex II ambiguities-FADH2 in the electron transfer system
Erratum in
-
Correction: Complex II ambiguities-FADH2 in the electron transfer system.J Biol Chem. 2024 Dec;300(12):108009. doi: 10.1016/j.jbc.2024.108009. Epub 2024 Dec 10. J Biol Chem. 2024. PMID: 39662301 Free PMC article. No abstract available.
Abstract
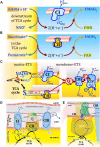
The prevailing notion that reduced cofactors NADH and FADH2 transfer electrons from the tricarboxylic acid cycle to the mitochondrial electron transfer system creates ambiguities regarding respiratory Complex II (CII). CII is the only membrane-bound enzyme in the tricarboxylic acid cycle and is part of the electron transfer system of the mitochondrial inner membrane feeding electrons into the coenzyme Q-junction. The succinate dehydrogenase subunit SDHA of CII oxidizes succinate and reduces the covalently bound prosthetic group FAD to FADH2 in the canonical forward tricarboxylic acid cycle. However, several graphical representations of the electron transfer system depict FADH2 in the mitochondrial matrix as a substrate to be oxidized by CII. This leads to the false conclusion that FADH2 from the β-oxidation cycle in fatty acid oxidation feeds electrons into CII. In reality, dehydrogenases of fatty acid oxidation channel electrons to the Q-junction but not through CII. The ambiguities surrounding Complex II in the literature and educational resources call for quality control, to secure scientific standards in current communications of bioenergetics, and ultimately support adequate clinical applications. This review aims to raise awareness of the inherent ambiguity crisis, complementing efforts to address the well-acknowledged issues of credibility and reproducibility.
Keywords: Complex II; coenzyme Q; electron transfer system; fatty acid oxidation; flavin adenine dinucleotide; succinate dehydrogenase; tricarboxylic acid cycle.
Copyright © 2023 The Author. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest E. Gnaiger is editor-in-chief of Bioenergetics Communications.
Figures




References
-
- Gnaiger E., Eleonor A.F., Norwahidah A.K., Ali A.R., Abumrad Nada A., Acuna-Castroviejo D., et al. MitoEAGLE Task Group. Mitochondrial physiology. Bioenerg. Commun. 2020;1:1–44.
-
- Murphy M.P., O’Neill L.A.J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018;174:780–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

